Cumene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
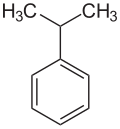
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cumene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 12 | |||||||||||||||
| Brief description |
colorless liquid with a sharp, aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 120.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.86 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−96 ° C |
|||||||||||||||
| boiling point |
152 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.49146 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data |
LD 50 oral rat: 1400 mg / kg |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cumene (according to IUPAC nomenclature: propan-2-ylbenzene , often also referred to as isopropylbenzene ) is an organic-chemical compound from the group of aromatic hydrocarbons . Due to the elegant conversion into phenol and acetone by the Hock process and its industrial distribution in the fifties, it became an important intermediate product in the chemical industry .
history
Cumene was discovered in the decarboxylation of cumic acid in 1840 . The first name proposed by the discoverer was cumene , they used the first word element of the Cum ynoic . Justus v. Liebig then suggested the name cumene , which from then on found its way into German literature.
Extraction and presentation
The technically exclusive method for the production of cumene is based on the catalytic alkylation of benzene with propene . The conversion takes place either in the liquid or gas phase . Friedel-Crafts systems or Brønsted acids and, more recently, acidic zeolites are mainly used as catalysts .
Liquid phase
In the liquid phase process, benzene is reacted with propene at 35–40 ° C. and a low propene pressure of around 7 bar in the presence of aluminum chloride (AlCl 3 ) to form cumene.
In addition, a process is known using hydrogen fluoride at 50-70 ° C. and likewise low propylene pressure. In acid-catalyzed alkylation, sulfuric acid is rarely and occasionally used.
Gas phase
The alkylation of benzene with propene in the gas phase is carried out at temperatures of 200-250 ° C. and pressures of 20-40 bar over phosphoric acid catalysts which are supported on silicon dioxide and contain boron trifluoride as a promoter . The entire reaction takes place in the fixed bed reactor .
The selectivities here reach 96–97% based on benzene and 91–92% based on propene. Only small amounts of di- and triisopropylbenzene and propylbenzene are produced as by-products . After working up by distillation , cumene is obtained in a purity of more than 99.5%.
The global production capacities for cumene in 2004 were more than 10.5 million tons per year.
properties
Cumene has a characteristic, aromatic odor, the odor threshold is 0.04–6.4 mg · m −3 . In many common organic solvents , e.g. B. ether and ethanol , cumene is soluble, but very sparingly soluble in water.
Physical Properties
Cumene is a colorless liquid that boils at 152 ° C under normal pressure . The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.05419, B = 1455.811 and C = −65.948 in the temperature range from 343.2 K to 426.5 K. Important thermodynamic parameters are given in the following table:
| property | Type | Value [unit] | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid Δ f H 0 gas |
−41.2 kJ mol −1 3.92 kJ mol −1 |
as a liquid as a gas |
| Enthalpy of combustion | Δ c H 0 liquid | −5215.44 kJ mol −1 | as a liquid |
| Heat capacity | c p | 215.4 J mol −1 K −1 (25 ° C) 1.79 J g −1 K −1 (25 ° C) |
as a liquid |
| Critical temperature | T c | 631 K | |
| Critical pressure | p c | 32.1 bar | |
| Critical density | ρ c | 2.32 mol·l −1 | |
| Enthalpy of evaporation | Δ V H | 41.2 kJ mol −1 | at 364 K |
| Enthalpy of fusion | Δ fus H | 7.326 kJ mol −1 | at the melting point |
Safety-related parameters
Cumene forms highly flammable vapor-air mixtures. The compound has a flash point of 31 ° C. The explosion range is between 0.8% by volume (40 g / m 3 ) as the lower explosion limit (LEL) and 6% by volume (300 g / m 3 ) as the upper explosion limit (UEL). The lower explosion point is 29 ° C. The limit gap width was determined to be 1.05 mm. This results in an assignment to explosion group IIA. The ignition temperature is 420 ° C. The substance therefore falls into temperature class T2.
use
Cumene is used as an intermediate in the production of phenol and acetone using the cumene hydroperoxide process (Hock process).
In rare cases it is also used as a solvent. Cumene is also used as an anti-knock agent in jet fuel. Compared to benzene, which can also be used, it has a much lower pour point of about −96 ° C.
safety instructions
Cumene is flammable and irritates the respiratory system. It can also irritate the skin (burning sensation / itching) and eyes, damage the liver and cause dizziness and drowsiness. The IARC classified cumene as a possible carcinogen in 2013.
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r Entry on cumene in the GESTIS substance database of the IFA , accessed on February 15, 2017(JavaScript required) .
- ↑ Entry on cumene. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Entry on Cumene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 98-82-8 or iso-propylbenzene ), accessed on September 15, 2019.
- ^ Christian Wiegand: Origin and interpretation of important organic trivial names. I. Benzene series hydrocarbons. In: Angewandte Chemie . 60 (4), 1948, pp. 109-111; doi: 10.1002 / anie.19480600407 .
- ↑ a b c d e Hans-Jürgen Arpe: Industrial organic chemistry - important preliminary and intermediate products . 6th edition. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 2007, ISBN 978-3-527-31540-6 , p. 380 ff .
- ↑ CB Williamham, WJ Taylor, JM Pignocco, FD Rossini: Vapor Pressures and Boiling Points of Some Paraffin, Alkylcyclopentane, Alkylcyclohexane, and Alkylbenzene Hydrocarbons. In: J. Res. Natl. Bur. Stand. (US), 35, 1945, pp. 219-244, doi: 10.6028 / jres.035.009 .
- ↑ a b c E. J. Prosen, R. Gilmont, FD Rossini: Heats of combustion of benzene, toluene, ethyl-benzene, o-xylene, m-xylene, p-xylene, n-propylbenzene, and styrene. In: J. Res. Natl. Bur. Stand. (US), 34, 1945, pp. 65-70. (PDF)
- ↑ a b K. Kishimoto, H. Suga, S. Syuzo: Calorimetric study of the glassy state. VIII. Heat capacity and relaxational phenomena of isopropylbenzene. In: Bull. Chem. Soc. Japan . 46, 1973, pp. 3020-3031, doi: 10.1246 / bcsj.46.3020 .
- ↑ a b C. Tsonopoulos, D. Ambrose: Vapor-Liquid Critical Properties of Elements and Compounds. 3. Aromatic hydrocarbons. In: J. Chem. Eng. Data . 40, 1995, pp. 547-558, doi: 10.1021 / je00019a002 .
- ↑ JL Hales, R. Townsend: Liquid Densities from 293 to 490 K of Nine Aromatic Hydrocarbons. In: J. Chem. Thermodyn. 4, 1972, pp. 763-772, doi: 10.1016 / 0021-9614 (72) 90050-X .
- ^ Emilio Cepeda, Cristina Gonzalez, Jose M. Resa: Isobaric vapor-liquid equilibrium for the cumene-phenol system. In: J. Chem. Eng. Data . 34, 1989, pp. 270-273, doi: 10.1021 / je00057a004 .
- ↑ a b c d E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ^ Siegfried Hauptmann : Organic chemistry. 2nd Edition. German publishing house for basic industry, Leipzig 1985, ISBN 3-342-00280-8 , p. 334.
- ↑ IARC Monograph 101 - Cumene, 2013
Web links
- Entry to cumene . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed December 18, 2012.