Glyoxylic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
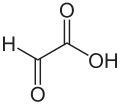
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Glyoxylic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 2 H 2 O 3 | |||||||||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 74.04 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| pK s value |
3.18; 3.32 |
|||||||||||||||||||||
| solubility |
easily in water, little in ethanol , diethyl ether and benzene |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Glyoxylic acid (also oxoacetic acid , glyoxalic acid , ethanolic acid or oxoethanoic acid ) consists of an aldehyde and a carboxy group . It represents an intermediate stage in the oxidation of glycolic acid to oxalic acid . Its salts are called glyoxylates.
Occurrence
Glyoxylic acid is found in young green leaves and unripe fruits (especially rhubarb , currants and gooseberries ). In glacial acetic acid glyoxylic acid is also present in small quantities.
Extraction and presentation
To obtain glyoxylic acid, dichloroacetic acid is hydrolyzed or oxalic acid is reduced electrolytically . Oxidation of glycolic acid or ozonolysis of maleic acid dimethyl ester and subsequent hydrolysis of the resulting glyoxylic acid methyl ester -methyl-hemi- acetal is also possible . The technical process of ozonolysis of maleic acid dimethyl ester proved to be uncontrollable and was discontinued in 2004. The industrially most important route to glyoxylic acid today is the oxidation of glyoxal with concentrated nitric acid .
use
Glyoxylic acid is used in the synthesis of allantoin , antibiotics , complexing agents , crop protection agents , vanillin , ethylvanillin and other chemical products. Reaction with cyclohexanone , subsequent elimination of water and catalytic dehydrogenation produces 2-coumaranone ( benzofuran -2 (3 H ) -one), a starting material for the preparation of the fungicide and strobilurin azoxystrobin .
properties
Glyoxylic acid crystallizes from an aqueous solution as glyoxylic acid hydrate (dihydroxyacetic acid). This connection is an exception to the Erlenmeyer rule .
Individual evidence
- ↑ Entry on GLYOXYLIC ACID in the CosIng database of the EU Commission, accessed on June 30, 2020.
- ↑ a b c d e f g Entry on glyoxylic acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 10, 2014.
- ↑ Dissociation Constants Of Organic Acids and Bases (600 compounds)
- ↑ chem.wisc.edu: pKa Data , Compiled by R. Williams (PDF, 78 kB).
- ↑ a b Entry on glyoxylic acid in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Hans-Jürgen Arpe: Industrial Organic Chemistry , 6th edition, 2007.
- ↑ Patent US5616733 : Preparation method for 2-coumaranone. Applied on June 7, 1995 , published April 1, 1997 , applicant: Societe Francaise Hoechst, inventors: Jean-Claude Vallejos, Alain Perrard, Yani Christidis, Pierre Gallezot.


