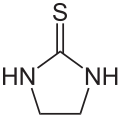
Ethylene thiourea
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethylene thiourea | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 6 N 2 S | |||||||||||||||
| Brief description |
colorless, crystalline solid with a slightly aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 102.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.4 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
203-204 ° C |
|||||||||||||||
| Vapor pressure |
0.2 h Pa (170 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
of particular concern : toxic for reproduction ( CMR ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ethylene thiourea , abbreviated ETU (from English Ethylenethiourea ), is a commercially available vulcanization accelerator and, moreover, a toxicologically important metabolite of the fungicides from the group of ethylene bisdithiocarbamates (e.g. Zineb , Maneb , Metiram ). The compound can also appear as a contaminant in these crop protection products .
Manufacturing
Ethylene thiourea is produced by reacting ethylenediamine (H 2 N – CH 2 –CH 2 –NH 2 ) with carbon disulfide (CS 2 ) to form dithiocarbamate , followed by a condensation reaction with ring closure and elimination of hydrogen sulfide (H 2 S).
application
The only significant application is as a vulcanization accelerator for chloroprene rubber (polychloroprene). In this function, ethylenethiourea accepts according to the classical view in the presence of metal oxides such as zinc oxide and magnesium oxide , the role of a sulfur donor ( sulfur donor ). However, it has also been proven that ETU binds to the polychloroprene via the nitrogen atoms during vulcanization. Ethylene thiourea is commercially available, preferably dust-free, either coated with oil or in a mixture with polymers ( patched ), e.g. B. Rhenocure ® NPV / C.
toxicology
In plant foods, ethylene thiourea can occur as a residue from the decomposition of fungicides from the class of ethylene bisdithiocarbamates (EBDC). Due to the toxicity and the suspicion of a carcinogenic effect, the limit value for ETU is shown to be correspondingly low at 0.05 ppm (BGBI Residue Maximum Quantity Ordinance of January 13, 2003). The analytical evidence according to the recommendation of the Federal Institute for Risk Assessment (BfR) from 2002 is carried out by gas chromatography with a sulfur- specific flame photometric detector . Detection with a mass spectrometer is also possible.
literature
- R. Musch, E. Rohde and H. Casselmann: Polychloroprene Crosslinking for Improved Aging Resistance . In: Kautsch. Rubber plastic 49: 340 (1996).
Web links
- Toxicological assessment of ethylene thiourea (PDF) from the professional association raw materials and chemical industry (BG RCI), accessed on May 1, 2018.
Individual evidence
- ↑ a b c d Entry on imidazolidine-2-thione in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b Data sheet imidazolidin-2-thione (PDF) from Merck , accessed on March 31, 2011.
- ↑ a b c Entry on imidazolidine-2-thione. In: Römpp Online . Georg Thieme Verlag, accessed on May 1, 2014.
- ↑ Entry on Imidazolidine-2-thione in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on October 19, 2015.
- ↑ Entry on ethylene thiourea in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Dissertation Jürgen Blanke: Model studies on the influence of temperature and 2-imidazolidinthione on the crosslinking of poly (chloroprene) . University of Hanover 1988.
- ↑ Federal Institute for Risk Assessment: Ethylenethiourea (ETU) ( Memento of the original from March 22, 2016 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 350 kB).

