Divinyl sulfide
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
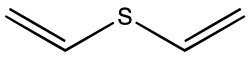
|
|||||||||||||
| General | |||||||||||||
| Surname | Divinyl sulfide | ||||||||||||
| Molecular formula | C 4 H 6 S | ||||||||||||
| Brief description |
colorless liquid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 86.15 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| density |
0.91 g cm −3 |
||||||||||||
| boiling point |
83-84 ° C (720 mmHg) |
||||||||||||
| solubility |
|
||||||||||||
| Refractive index |
1.5018 (20 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||
Divinyl sulfide is a chemical compound from the group of organosulfur compounds .
Occurrence
Divinyl sulfide occurs naturally in wild garlic .
Extraction and presentation
Divinyl sulfide was first synthesized in 1920 by OB Helfrich and EE Reid. In studying the reaction of mustard gas with sodium ethoxide , they isolated the compound in place of the expected 2,2'-diethoxydiethyl sulfide.
Later, when investigating the dehydrochlorination of mustard gas with alcoholic potassium hydroxide , SH Bales and SA Nickelson showed that the main product of this reaction is also divinyl sulfide in yields of 26 to 35%, depending on the concentration of the water in the reaction mixture. Many other synthetic methods are known today.
A method with high yield is the synthesis by reaction of acetylene with hydrogen sulfide . The appearance of divinyl sulfide in acetylene during its manufacture from calcium carbide can be attributed to the presence of traces of calcium sulfide in commercially available calcium carbide.
properties
Divinyl sulfide is an unstable colorless liquid with a slight odor that is sparingly soluble in water. It is decomposed by concentrated sulfuric acid and turns red.
Individual evidence
- ↑ a b c d e f g h i j B. A. Trofimov, SV Amosova: Divinyl Sulfide: Synthesis, Properties, and Applications. In: Sulfur reports. 3, 2007, p. 323, doi : 10.1080 / 01961778408082463 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Robert Ebermann, Ibrahim Elmadfa: Textbook food chemistry and nutrition . Springer-Verlag, 2008, ISBN 978-3-211-49348-9 , pp. 388 ( limited preview in Google Book search).
- ^ K. Paech, MV Tracey: Modern Methods of Plant Analysis / Modern Methods of Plant Analysis . Springer Science & Business Media, 2013, ISBN 978-3-642-64961-5 , p. 693 ( limited preview in Google Book search).
