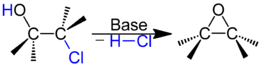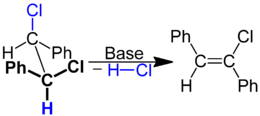Dehydrochlorination
| Dehydrochlorination |
| Formation of an alkene by β-elimination |
| Formation of an oxirane by 1,3-dehydrochlorination |
| Formation of a thiirane by 1,3-dehydrochlorination |
| Formation of an oxirane by 1,3-dehydrochlorination |
| Formation of a vinyl chloride derivative by 1,2-dehydrochlorination |
| Formation of a ketene by 1,3-dehydrochlorination |
In organic chemistry, dehydrochlorination is an elimination reaction - often a β-elimination reaction or β-elimination - or an intramolecular substitution reaction . By splitting off one hydrogen and one chlorine atom from the same compound, an alkene is formed (in the 1,2-position of the hydrogen and chlorine atom), a cyclopropane derivative (in the 1,3-position of the hydrogen and chlorine atom) or a heterocyclic compound. In dehydrochlorination, hydrogen chloride is split off under the action of bases. As bases to use alkali metal hydroxides , amines , alkylamides or heterocyclic nitrogen compounds such. B. 1,5-diazabicyclo [4.3.0] non-5-ene (DBN).
Chlorovinyl compounds can be synthesized from 1,2- or 1,1-dichloro compounds under the action of alcoholic potassium hydroxide solution. With a suitable substitution pattern and harsher reaction conditions, a second dehydrochlorination takes place with the formation of alkynes.
Further examples of dehydrochlorination under the action of a base:
- Formation of oxiranes from β-chloroalkanols
- Formation of oxetanes from γ-chloroalkanols
- Formation of aziridines from β-chloramines
- Formation of azetidines from γ-chloramines
- Formation of thiiranes from β-chlorothioalkanols
- Formation of ketenes from carboxylic acid chlorides with a hydrogen atom on the α-carbon atom
The formation of the reactive and unstable didehydrobenzene (an aryne ) from chlorobenzene is also a dehydrochlorination.
Reaction mechanism
A distinction is made between monomolecular elimination (E1) and bimolecular elimination (E2). There is also the carbanion or E1cB mechanism according to which such β-eliminations can take place.
Individual evidence
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 2: Cm-G. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1981, ISBN 3-440-04512-9 , p. 883.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 258, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, pp. 558-563, ISBN 3-342-00280-8 .
- ↑ Organikum , Wiley-VCH Verlag GmbH, 23rd edition, 2009, pp. 280-281, ISBN 978-3-527-32292-3 .
- ↑ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, p. 155, ISBN 3-211-81060-9 .
- ^ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, pp. 136–151, ISBN 3-211-81060-9 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 229, ISBN 3-342-00280-8 .