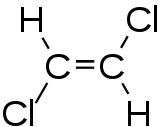
1,2-dichloroethene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cis -1,2-dichloroethene (left), trans -1,2-dichloroethene (right) | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,2-dichloroethene | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 2 Cl 2 | ||||||||||||||||||
| Brief description |
highly flammable, colorless liquid with an ethereal odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 96.94 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
1,2-Dichloroethene is a chemical compound from the group of chlorinated alkenes that occurs in the form of two cis-trans isomers .
Extraction and presentation
1,2-dichloroethene can be produced by the chlorination of acetylene or by reducing 1,1,2,2-tetrachloroethane .
It is also a by-product of the production of other C 2 chlorinated hydrocarbons such as trichlorethylene .
Occurrence
1,2-Dichloroethene is naturally formed under anaerobic conditions with corresponding environmental pollution from tetrachloroethene and trichloroethene as a result of reductive dehalogenation by autochthonous microbes, with 1,1-dichloroethene also being formed in small quantities .
properties
1,2-dichloroethene is a highly volatile, highly flammable liquid with a very strong odor and can therefore be noticed even in the smallest doses (≈ 15 ppm ). The unstabilized product can polymerize.
use
1,2-dichloroethene is used as a solvent for waxes , resins , fats , lacquers and polymers , and as a starting material for the production of other solvents and chlorinated compounds.
safety instructions
1,2-dichloroethene is highly flammable; the vapors have a narcotic effect and form an explosive mixture with air (flash point 2 ° C, ignition temperature 460 ° C).
See also
Individual evidence
- ↑ a b c d e f g h Entry on trans-1,2-dichloroethene in the GESTIS substance database of the IFA , accessed on February 22, 2017 (JavaScript required)
- ↑ a b c d e f Entry on cis-1,2-dichloroethene in the GESTIS substance database of the IFA , accessed on February 22, 2017 (JavaScript required)
- ↑ a b c d e f g h Entry on cis / trans-1,2-dichloroethene in the GESTIS substance database of the IFA , accessed on February 22, 2017(JavaScript required) .
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-154.
- ↑ Entry on 1,2-dichloroethylene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 540-59-0 or 1,2-dichloroethene sym. (Cis and trans) ), accessed on October 3, 2019.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-21.
- ↑ Petra Koziollek; Cometabolic degradation of cis-1,2-dichloroethene and vinyl chloride by bacteria utilizing ethene; ISBN 978-3-8167-5520-3