Propargyl alcohol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Propargyl alcohol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 4 O | |||||||||||||||
| Brief description |
colorless liquid with a slight geranium-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 56.06 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.948 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−48 ° C |
|||||||||||||||
| boiling point |
115 ° C |
|||||||||||||||
| Vapor pressure |
10 hPa (20 ° C) |
|||||||||||||||
| pK s value |
13.6 (25 ° C) |
|||||||||||||||
| solubility |
miscible with water, ethanol, diethyl ether, pyridine, benzene, trichloromethane |
|||||||||||||||
| Refractive index |
1.432 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 2 ml m −3 or 4.7 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Propargyl alcohol (according to IUPAC nomenclature: prop-2- yn -1-ol ) is an organic-chemical compound from the group of alkynols . It is an intermediate product in the chemical industry .
Extraction and presentation
Propargyl alcohol is produced on an industrial scale by the Reppe process (ethynylation). Thereby responding acetylene in large excess with formaldehyde under copper (I) acetylide - catalysis at temperatures from 95 to 150 ° C and pressures of from 2 to 3: propargyl alcohol.
properties
The flammable, colorless liquid has a characteristic odor. With a flash point of 33 ° C, explosive mixtures can form with the air above this temperature. The explosion range is between 2.8% by volume (66 g / m 3 ) as the lower explosion limit (LEL) and 78% by volume (1817 g / m 3 ) as the upper explosion limit (UEL). The steam is heavier than air. The ignition temperature is 365 ° C. The substance therefore falls into temperature class T2. Propargyl alcohol can be polymerized under the influence of heat, light, oxidizing agents and peroxides . Violent reactions with the development of heat can take place with oxidizing agents. Exceeding the occupational exposure limit cannot be determined from the smell.
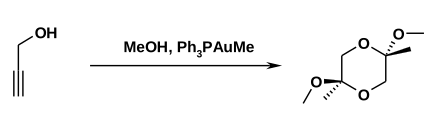
Propargyl alcohol, in the presence of gold catalysts and methanol to a cyclic dimeric bisketal be implemented, eluting with 93% yield the trans - isomer is formed.
use
Propargyl alcohol is used, among other things, as a corrosion inhibitor for steel (against attack by acids), as a stabilizer for chloroalkanes and as an intermediate for organic synthesis.
Web links
Individual evidence
- ↑ a b c d Entry on prop-2-yn-1-ol. In: Römpp Online . Georg Thieme Verlag, accessed on January 19, 2019.
- ↑ a b c d e f g h i j k Entry on 2-propyn-1-ol in the GESTIS substance database of the IFA , accessed on January 19, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dissociation Constants of Organic Acids and Bases, pp. 8-43.
- ↑ a b data sheet Propargyl alcohol from Sigma-Aldrich , accessed on January 19, 2019 ( PDF ).
- ↑ Entry on Prop-2-yn-1-ol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 19, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 107-19-7 or propargyl alcohol ), accessed on November 2, 2015.
- ↑ Nicole Schoedel: Process for the production of propargyl alcohol. In: Google Patents. Linde AG, January 27, 1999, accessed December 9, 2018 (German, English).
- ^ E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ JH Teles, S. Brode, M. Chabana: Cationic Gold (I) Complexes: Highly Efficient Catalysts for the Addition of Alcohols to Alkynes in Angew. Chem. , Int. Ed. 37 (1998) 1415-1418, doi : 10.1002 / (SICI) 1521-3773 (19980605) 37:10 <1415 :: AID-ANIE1415> 3.0.CO; 2-N .