Vinyl ester resins
Vinyl ester resins ( abbreviation VE resins, also called phenacrylate resins ) are synthetic resins which, when cured, form thermosetting plastics of high strength and chemical resistance . In addition to epoxy resins and unsaturated polyester resins (UP resins), vinyl ester resins are used in the manufacture of glass fiber reinforced plastics . VE resins consist of a prepolymer with two or more acrylate or methacrylate groups (“vinyl ester”) and a monomer with a vinyl group , usually styrene . VE resins differ from UP resins, since in VE resins the reactive C = C double bonds only occur at the end of the prepolymer and, when the resins harden, lead to less close-meshed crosslinking of the thermosets. VE resins are among the reaction resins , as no condensate is released during curing.
Manufacturing

Vinyl ester
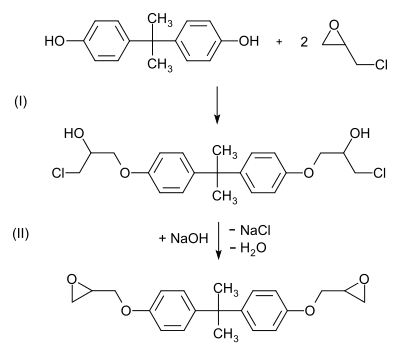
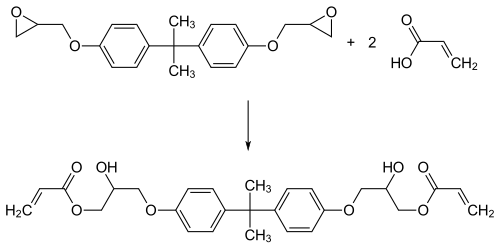
Vinyl esters are produced as prepolymers by esterifying epoxy resins with acrylic acid or methacrylic acid. In addition to novolak epoxy resins , bisphenol A diglycidyl ethers are usually used as epoxides . Typical is the conversion of bisphenol A with epichlorohydrin to form epoxide and the subsequent esterification with acrylic acid.
Epichlorohydrin is added to bisphenol A and bis (3-chloro-2-hydroxy-propoxy) bisphenol A is formed. The bis-epoxide is then formed in a condensation reaction with a stoichiometric amount of sodium hydroxide . In an addition reaction , an ester with a vinyl group is formed from the epoxy groups of the bisphenol A diglycidyl ether and acrylic acid.
Vinyl monomer
The reaction product is then dissolved, for example, in styrene with a mass content of 35 to 45%. Additives such as accelerators for hardening ( siccatives ) or paraffins to inhibit the evaporation of the monomer during hardening can also be added to the more or less viscous product .
Hardening
After addition of a peroxide - initiator is a free-radical copolymerization of a styrene and the unsaturated prepolymer. Compounds such as dibenzoyl peroxide and methyl ethyl ketone peroxide are used as initiators . In addition to hot hardening, cold hardening is possible by adding accelerators. UV curing is also possible with photoinitiators .
The curing of novolak vinyl esters is analogous. For thermosets with a high application temperature, diisocyanates are also used in novolak vinyl esters , which allow additional crosslinking between the hydroxyl groups of the prepolymer with the formation of urethane groups . These resins are called vinyl ester urethane resins (VEU resins).
properties
The hardened resin allows continuous temperatures of up to 125 ° C in use and is resistant to 37% hydrochloric acid and 50% sodium hydroxide solution. Compared to the brittle thermosetting plastics made from UP resins, the lower degree of cross-linking leads to a material that is quite impact-resistant and somewhat more flexible. The elongation at break is between 5 and 6%.
application
- Chemical apparatus construction: tanks, pipes, cooling towers
- Automotive construction: oil sump pans, cylinder head covers, large-area body parts
- Sports: boat masts, ski poles
Individual evidence
- ↑ a b c Gottfried Wilhelm Ehrenstein: fiber composite plastics , Carl Hanser, Munich, 2006, p. 60f. (Limited preview) .
- ^ A b Wolfgang Kaiser: Kunststoffchemie für Ingenieure , 3rd edition, Carl Hanser, Munich, 2011, p. 435 f.
- ↑ Entry on vinyl ester resins. In: Römpp Online . Georg Thieme Verlag, accessed on June 7, 2014.