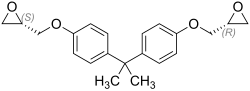
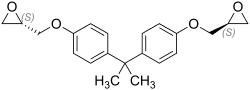
Bisphenol A diglycidyl ether
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structure without showing the stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Bisphenol A diglycidyl ether | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 21 H 24 O 4 | |||||||||||||||||||||
| Brief description |
colorless, odorless liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 340.41 g mol −1 | |||||||||||||||||||||
| Physical state |
viscous |
|||||||||||||||||||||
| density |
1.16 g cm −3 |
|||||||||||||||||||||
| Melting point |
8-12 ° C |
|||||||||||||||||||||
| Vapor pressure |
negligible |
|||||||||||||||||||||
| solubility |
practically insoluble in water (0.7 mg l −1 at 25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Bisphenol A diglycidyl ether (abbreviated to BADGE or DGEBA by D i g lycidyl e ther of B isphenol A ) is a chemical compound used as monomer for the production of epoxy resins and phenolic resins is utilized. It is a derivative of bisphenol A , which is also used in the manufacture of epoxy resins due to its crosslinking ability .
synthesis
Bisphenol A diglycidyl ether is made from bisphenol A with an excess of epichlorohydrin . In a two-stage reaction, epichlorohydrin is first added to bisphenol A and then the bis-epoxide is formed from this with a stoichiometric amount of sodium hydroxide solution .
Chemical properties
Stereochemistry
Bisphenol A diglycidyl ether is generally present as a mixture of stereoisomers .
The ( R , R ) form forms a racemate with the ( S , S ) form, the ( R , S ) form is an achiral meso form . All three stereoisomeric forms are present in the mixture.
use
The structure of many standard epoxies is based on diglycidyl ethers, including BADGE. Epoxy resins are sensitive to high temperatures, traces of acids and easily add thiols and amines. The most common hardeners for epoxy resins are polyamines , aminoamides and phenolic compounds .
Coatings based on BADGE-containing epoxy resins are used for the inner lining of cans and screw caps ( twist-off lids ). BADGE could leak into food from these coatings as an unreacted monomer.
safety instructions
BADGE is listed as a Group 3 chemical by the International Agency for Research on Cancer (IARC), which means that it is "non-classifiable for its carcinogenicity to humans." Since the 1990s there have been concerns about carcinogenicity , mainly because, as mentioned, BADGE comes into contact with food.
Similar to higher concentrations of the chlorinated precursor product of BADGE have been found in marine mammals than of persistent organic pollutants such as polybrominated diphenyl ethers or perfluorooctanesulfonate .
Bisphenol A diglycidyl ether was included in the EU's ongoing action plan ( CoRAP ) in 2013 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of bisphenol A diglycidyl ether were concerns about consumer use , high (aggregated) tonnage and widespread use as well as the dangers arising from a possible assignment to the group of CMR substances and as a potential endocrine disruptor . The re-evaluation has been running since 2015 and is carried out by Denmark . In order to be able to reach a final assessment, further information was requested.
See also
Individual evidence
- ↑ a b c d e f g Entry on 2,2-bis (4- (glycidyloxy) phenyl) propane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on 2,2 ′ - [(1-methylethylidene) bis (4,1-phenyleneoxymethylene)] bisoxirane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can use the harmonized classification and labeling expand .
- ↑ a b Walfried Rauter, Gerald Dickinger, Rudolf Zihlarz, Josef Lintschinger: Determination of bisphenol A diglycidyl ether (BADGE) and its hydrolysis products in canned oily foods from the Austrian market . In: Journal of Food Control and Research A . 208, No. 3, March 1999, pp. 208-211. doi : 10.1007 / s002170050404 .
- ↑ Federal Environment Agency: Guideline for the hygienic assessment of organic coatings in contact with drinking water (coating guideline) ( Memento from April 26, 2014 in the Internet Archive ) (PDF; 241 kB) from November 30, 2010, accessed on May 31, 2013.
- ↑ Martin Forrest: Coatings and Inks for Food Contact Materials (= RAPRA review reports . Vol. 16, No. 6 ). iSmithers Rapra Publishing, 2005, ISBN 978-1-84735-079-4 , pp. 8 .
- ↑ Federal Office for Consumer Protection and Food Safety (BVL), ed., Official collection of investigation procedures according to § 35 LMBG, Beuth: Berlin, (loose-leaf collection); L 00.00-51; BVL online method collection , as of December 9, 2007.
- ^ List of Classifications - IARC Monographs on the Identification of Carcinogenic Hazards to Humans. IARC , accessed July 14, 2020 .
- ↑ Monograph 71 - Bisphenol A diglycidyl ether , International Agency for Research on Cancer
- ↑ Jingchuan Xue, Kurunthachalam Kannan: Novel Finding of Widespread Occurrence and Accumulation of Bisphenol A Diglycidyl Ethers (BADGEs) and Novolac Glycidyl Ethers (NOGEs) in Marine Mammals from the United States Coastal Waters. In: Environmental Science & Technology . 2016, doi: 10.1021 / acs.est.5b04650 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 2,2 ′ - [(1-methylethylidene) bis (4,1-phenyleneoxymethylene)] bisoxirane , accessed on March 26, 2019.