Pentines
| Pentines | ||||||||
| Surname | Pent-1-in | Pent-2-in | 3-methylbut-1-yne | |||||
| other names | Propyl acetylene | 1-methyl-2-ethylacetylene | Isopropyl acetylene, isopentine | |||||
| Structural formula |  |
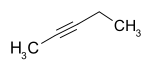 |
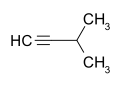
|
|||||
| CAS number | 627-19-0 | 627-21-4 | 598-23-2 | |||||
| PubChem | 12309 | 12310 | 69019 | |||||
| Molecular formula | C 5 H 8 | |||||||
| Molar mass | 68.12 g mol −1 | |||||||
| Physical state | liquid | |||||||
| Brief description | colorless liquid | |||||||
| Melting point | −106 ° C | −109 ° C | −90 ° C | |||||
| boiling point | 39-41 ° C | 55-57 ° C | 29.5 ° C | |||||
| density | 0.69 g cm −3 | 0.71 g cm −3 | 0.666 g cm −3 (20 ° C) | |||||
| solubility | 1.05 g l −1 in water | insoluble in water | ? | |||||
|
GHS labeling |
|
|
|
|||||
| H and P phrases | 225 | 225-315-319-335 | 224-315-319-335 | |||||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||||
| 210-223-403 + 235 |
210-302 + 352-304 + 340 305 + 351 + 338-403 + 235 |
210-240-243-261-280 302 + 352 305 + 351 + 338-403 + 235 |
||||||
In chemistry, the pentynes form a group of unsaturated aliphatic hydrocarbons with the general empirical formula C 5 H 8 and a C≡C triple bond . They are therefore one of the alkynes .
There are three isomers :
- 1-pentin
- 2-pentin
- 3-methyl-1-butyne
All three isomers are volatile, flammable liquids under normal conditions and almost insoluble in water. The isomers are very reactive due to their C≡C triple bond like most alkynes and can, for. B. polymerize easily or enter into addition reactions .
Manufacture and extraction
In the preparation of the pentynes, one can start from the corresponding alkene compounds, which are first converted into the corresponding vicinal dibromides by addition of bromine . A dehydrohalogenation under basic conditions leads to the corresponding Pentinen. The synthesis of 1-pentyne is based on 1-pentene. The intermediate dibromide is dehydrohalogenated in the presence of potassium carbonate at high temperatures. 2-pentyne is produced in a similar manner, where the dehydrohalogenation takes place in alcoholic potassium hydroxide solution. The synthesis of 3-methyl-1-butyne goes from 3-methyl-1-butene from which first at -60 ° C brominated is. The resulting dibromide is dehydrohalogenated in liquid ammonia using sodium amide.
Individual evidence
- ↑ a b c d e Data sheet 1-Pentin (PDF) from Merck , accessed on February 21, 2010.
- ↑ a b c d e Data sheet 2-Pentin (PDF) from Merck , accessed on February 21, 2010.
- ↑ a b c H. N. Miller, KW Greenlee, JM Derfer, CE Boord: "Mono- and Di-Alkylacetylenes from Vicinal Dihalides and Sodium Amide in Liquid Ammonia", in: J. Org. Chem. , 1954 , 19 , p. 1882 -1888 ( doi : 10.1021 / jo01377a003 ).
- ↑ a b c 3-Methyl-1-butyne data sheet from Acros, accessed September 30, 2012.
- ^ HH Guest: "Rearrangements of the Triple Bond", in: J. Am. Chem. Soc. , 1928 , 50 , pp. 1744-1746 ( doi : 10.1021 / ja01393a036 ).
- ↑ ML Sherrill, ES Matlack: “Additional Data on the cis and trans Isomers of Pentene-2”, in: J. Am. Chem. Soc. , 1937 , 59 , pp. 2134-2138 ( doi : 10.1021 / ja01290a014 ).

