Brachytherapy
Brachytherapy (as "irradiation from within" derived from ancient Greek βραχύς brachys , German 'short, near' ), also internal radiation therapy, therapy with enclosed radiation sources or short-distance therapy , is a form of radiation therapy (usually with gamma radiation ), in which an enclosed radioactive radiation source is placed within or in the immediate vicinity of the area to be irradiated in the body. Brachytherapy is widely used for cancers of the cervix, prostate, breast, and skin. It can also be used to treat tumors in many other parts of the body. Brachytherapy can be used alone or in conjunction with other forms of therapy, for example surgery, external radiation therapy (teletherapy, percutaneous radiation, EBRT, external beam radiotherapy ) and chemotherapy.
Brachytherapy is not counted as a nuclear medicine procedure, although like this (see radionuclide therapy ) it uses the radiation emitted by radionuclides.
In contrast to external radiation therapy, i.e. irradiation from the outside, in which high-energy rays are directed from outside the body onto the target area, in brachytherapy the radiation sources are placed directly at the location of the cancerous tumor. A key feature of brachytherapy is that the radiation effect only affects a very limited area around the radiation source. Therefore, the radiation exposure for healthy tissue and tissue that is further away from the radiation sources is greatly reduced. In addition, the radiation sources maintain their exact position in relation to the tumor, even if the patient moves or if the tumor moves within the body during treatment. These features offer an advantage of brachytherapy over external radiation: the tumor can be treated with high-dose localized radiation, while at the same time reducing the likelihood of unnecessary damage to healthy surrounding tissue.
The implementation of a brachytherapy treatment series requires a shorter treatment time than other radiotherapy procedures. This can help reduce surviving cancer cells having fewer opportunities to divide and grow in the intervals between each dose of radiation therapy. Compared to external radiation therapy, patients usually need to visit the radiation clinic less frequently and treatment can often be carried out on an outpatient basis. Due to the good tolerance, this form of treatment is a relief for many patients.
Brachytherapy is an effective treatment option for many types of cancer. Therapy results have shown that the cure rates for cancer are comparable or better between brachytherapy, surgery and external radiation therapy. In addition, the risk of side effects is significantly lower.
history
Brachytherapy dates back to the 1901 return (shortly after the discovery of radioactivity by Becquerel 1896) - Pierre Curie made Henri-Alexandre Danlos introduce a radiation source into a tumor the proposal. It turned out that the radiation caused the tumor to shrink. Independently of this, Alexander Graham Bell also suggested using radioactivity in this way. In the early 20th century, the way for application techniques of brachytherapy (radium) was paved by Danlos at the Curie Institute in Paris and by Robert Abbe at St. Luke's Hospital in New York.
After the initial interest in brachytherapy in Europe and the US, its use declined in the mid-20th century. The reason was the radiation exposure for the medical professionals when handling the radiation sources manually.
The development of remote-controlled reloading systems (the reloading process is usually called afterloading ) and the use of new radiation sources in the 1950s and 1960s reduced the risk of unnecessary radiation exposure for doctor and patient.
In the afterloading process, an empty, tubular applicator is pushed into the target volume (e.g. the uterus) before the actual therapy and, after checking the position, a radioactive preparation (e.g. 137 cesium) is loaded. The specimen is at the tip of a steel wire that is gradually pulled back and forth under computer control. After the pre-calculated time, the source is pulled back into a safe and the applicator is removed.
This and recent developments in three-dimensional imaging, computerized treatment planning systems, and afterloading devices have made brachytherapy a safe and effective treatment for many cancers today.
species
Brachytherapy can be subdivided and defined in more detail in different ways: With regard to the placement of the radiation sources in the target area of the treatment or the dose rate or intensity of the radiation that is directed at the tumor, as well as the duration of the radiation.
Placement of the radiation sources
There are two main forms of treatment for the placement of the radiation source: interstitial brachytherapy and contact brachytherapy .
- In interstitial brachytherapy, the radiation sources are introduced directly into the tumor tissue using rigid needles, catheters or tubes, e.g. into the prostate or breast.
- Contact brachytherapy means placing the radiation source (e.g. by afterloading) in an area next to the tumor tissue. It can be a body cavity (for example, cervix, uterus, or vagina - intracavitary brachytherapy), a body lumen (such as the trachea or esophagus - intraluminal brachytherapy), or an external location (for example, the skin - surface brachytherapy). A radiation source can also be placed permanently (as permanent implantation) in the form of implanted seeds (see below) or in blood vessels ( intravascular brachytherapy) for the treatment of coronary in-stent restenosis.
Dose rate
In brachytherapy, the dose rate (sometimes also the dose rate ) relates to the intensity of the radiation emitted into the surrounding medium. It is given in gray per hour (Gy / h).
- LDR brachytherapy (low dose rate brachytherapy) means the introduction of radiation sources with a low dose rate. This is up to 2 Gy / h. LDR brachytherapy is commonly used for cancers of the oral cavity, oropharynx, sarcoma and prostate cancer.
- MDR brachytherapy (medium dose rate brachytherapy) is characterized by a medium dose rate (2–12 Gy / h).
- HDR brachytherapy (high dose rate brachytherapy): The dose rate is over 12 Gy / h. The most common uses of HDR brachytherapy are found in cervical, esophageal, lung, breast and prostate cancers. Many HDR treatments are performed on an outpatient basis, but this depends on the location of the tumor being treated.
- PDR brachytherapy (pulsed dose rate brachytherapy) means that short pulses of radiation are given, usually once an hour. The aim is to mimic the overall intensity and effectiveness of LDR treatment. Typical tumor diseases that are treated with PDR brachytherapy are gynecological and ENT tumors.
Duration of dose delivery
The introduction of radioactive sources into the target area can be temporary or permanent .
- Temporary brachytherapy means that the radiation sources are introduced for a specified time (usually a few minutes or hours) and then removed again. The specific duration of treatment depends on many different factors such as the required intensity of the dose delivery and the type, size and position of the tumor. With LDR and PDR brachytherapy, the source typically remains in place for up to 24 hours before it is removed, while with HDR brachytherapy, treatment lasts a few minutes.
- Permanent brachytherapy , also known as seed implantation, means the introduction of small radioactive seeds or pellets (about the size of a grain of rice) into the tumor or the treatment site, where they remain permanently until the activity drops. The radiation level emanating from the sources then drops to almost zero over the course of weeks or months. The inactive seeds remain at the treatment site with no lasting effect. Permanent brachytherapy is most commonly used in the treatment of prostate cancer .
Clinical Applications
Brachytherapy is widely used to treat cervical, prostate, breast, and skin cancers.
Brachytherapy can also be used to treat tumors of the brain, eyes, head and neck region (lips, floor of the mouth, tongue, nasopharynx and oropharynx), the respiratory tract (windpipe and bronchi), the digestive tract (esophagus, bile, bile ducts, Rectum, anus), the urinary tract (bladder, urethra, penis), the female genital tract (uterus, vagina, vulva) and soft tissues (sarcomas).
Since the radiation sources for the treatment can be positioned within the tumor or very close to it, brachytherapy makes it possible to give a high radiation dose to a small volume. The radiation sources also keep their positions when the patient moves or when the tumor moves within the body. This enables doctors to achieve a high level of dose compliance - that is, to ensure that the entire tumor receives the optimal dose of radiation. This also reduces the risk of damage to healthy tissue and organs around the tumor, which increases the chances of healing and the maintenance of organ function.
The use of HDR brachytherapy makes it possible to reduce the overall duration of the treatment compared to external radiation. Compared to EBRT (External Beam Radio Therapy), patients treated with brachytherapy have to attend fewer radiotherapy appointments, which is why the total treatment time is shorter. Many brachytherapy treatments are performed on an outpatient basis. For working patients, for older patients or also for patients who do not live near a clinic, this can be an important advantage that enables them to have radiation treatment carried out on themselves. Shorter treatment times and outpatient methods can also help improve the efficiency of clinics.
Brachytherapy can be used to cure cancer in cases of small or locally advanced tumors, provided that the cancer has not metastasized (spread). In suitable cases, brachytherapy for primary tumors often represents an approach comparable to surgery, with the same probability of a cure with similar side effects. In the case of locally advanced tumors, however, surgery does not generally offer the best chance of recovery and is often technically not feasible. In these cases, radiation therapy (including brachytherapy) offers the only chance of recovery. In more advanced stages of the disease, brachytherapy can be used as a palliative treatment to relieve symptoms and bleeding.
If the tumor is not easily accessible, or if it is too large to ensure optimal dose distribution for the treatment area, brachytherapy can be used in conjunction with other treatments, such as external radiation or surgery. A combination of brachytherapy in conjunction with chemotherapy alone is rare.
cervical cancer
The use of brachytherapy in the treatment of early or localized cervical cancer is common and standard in many countries. For cervical cancer, either LDR, PDR, or HDR brachytherapy can be used. When used in conjunction with external radiation therapy, brachytherapy can produce better results than external radiation alone. Brachytherapy is so precise that a high dose of radiation can be aimed specifically at the cervix, which minimizes radiation exposure to neighboring tissues and organs. The chances of staying disease-free (tumor-free survival) and overall survival rates are similar for LDR, PDR, and HDR treatments. A main advantage of HDR treatment, however, is that any dose can be given on an outpatient basis and in a short time, which is much more pleasant for many patients.
Prostate cancer
To treat prostate cancer, brachytherapy can be used as permanent implantation of LDR seeds or as temporary HDR brachytherapy.
Permanent seed implantation is suitable for patients with localized tumors and a good prognosis. It has been shown to be highly effective in preventing the disease from coming back. The survival rate is similar to that of external radiation therapy or surgery (radical prostatectomy ), but is less associated with side effects such as impotence and incontinence. The procedure can be done quickly, and usually patients can go home on the day of treatment and go back to their normal routine two to three days later. Permanent implantation of seeds is usually less invasive compared to surgical removal of the prostate.
Temporary HDR brachytherapy is a newer approach to prostate cancer treatment, but is currently less common than seed implantation. It offers an alternative method of applying high-dose radiation that is based on the shape of the tumor in the prostate while protecting the surrounding tissue from radiation exposure. HDR brachytherapy is mainly used to deliver an additional dose to external radiation therapy (“saturation”, “boost”). HDR brachytherapy as a saturation for prostate cancer can also mean that the treatment period for the radiation is shortened compared to the use of external radiation therapy alone.
Breast cancer
For women who have undergone breast-conserving surgery or mastectomy, radiation therapy is a standard of care. It is an integral part of breast-conserving therapy. Brachytherapy can be used after an operation, before chemotherapy or in the case of an advanced breast tumor. Temporary HDR brachytherapy is commonly used to treat breast cancer. Postoperatively, brachytherapy can be used as a saturation (boost) following external irradiation of the entire breast. Brachytherapy has recently been used solely with a technique known as APBI (accelerated partial breast irradiation), with radiation being emitted only to the immediate vicinity of the original tumor.
The main advantage of breast brachytherapy compared to external radiation is that the tumor can be precisely irradiated at a high dose while sparing healthy breast tissue and the underlying ribs and lungs. An APBI can usually be completed within a week. The option of brachytherapy can be particularly important for working women, older patients or women who do not live near a clinic. The duration of treatment is short compared to EBRT; EBRT often requires more clinic visits over a period of one to two months.
The follow-up (up to six years) of breast cancer patients treated with brachytherapy showed excellent local control. In breast brachytherapy, radiation oncologists apply flexible plastic tubes (catheters) or a balloon to the chest. Twice a day on a set number of days, the catheter or balloon is connected to a brachytherapy device (also known as an HDR afterloader) and the radiation is safely and effectively delivered to the surgical site under computer control. The irradiation takes only a few minutes each time. At the end, the catheters or balloon are removed. The effectiveness of this treatment compared to external radiation therapy (EBRT) with a duration of three to eight weeks is currently being investigated.
Skin cancer
For non-melanoma-like skin cancer such as basal cell carcinoma and squamous cell skin cancer ( spinalioma ), HDR brachytherapy is an alternative treatment to surgery. This is particularly important for cancers of the nose, ears, eyelids or lips, where surgical interventions can cause disfigurement or a make expensive reconstruction necessary. In order to ensure close contact between the radiation source (s) and the skin, different applicators can be used which correspond to the curve of the skin surface and enable precise delivery of the optimal radiation dose. Brachytherapy achieves good clinical results with excellent cosmetic results in skin cancer. Clinical studies with up to five years of follow-up observations show that brachytherapy is highly effective in terms of local control and is comparable to the effectiveness of external radiation therapy. Treatment times are generally short, which is much more comfortable for the patient. It is believed that brachytherapy could become a standard treatment for skin cancer in the near future.
Liver cancer and liver metastases
The use of brachytherapy for liver cancer, liver metastases, but also metastases in many other abdominal or thoracic locations is relatively new. The irradiation takes place in the form of HDR brachytherapy in which image-controlled ablation catheters are introduced into the tumor through the skin. A detailed description can be found in the article CT-guided, interstitial brachytherapy .
Other areas of application
Brachytherapy can be used in the treatment of in-stent coronary restenosis. To do this, catheters are placed in the blood vessels through which the radiation sources are introduced and removed again. Brachytherapy has also been investigated for the treatment of peripheral vascular stenosis. It has also been considered for the treatment of atrial fibrillation.
Side effects
The likelihood and type of possible acute, subacute, or long-term side effects associated with brachytherapy will depend on the location of the tumor being treated and the type of brachytherapy used.
Acute
Acute side effects associated with brachytherapy include localized swelling, bleeding, discharge, or discomfort in the implantation area. In general, these subside within a few days after the end of treatment.
Brachytherapy treatment for cervical or prostate cancer can cause acute and transient urinary symptoms such as urinary retention, urinary incontinence, or painful urination (dysuria). Temporary increased stool frequency, diarrhea, constipation or minor rectal bleeding may also occur. Acute and subacute side effects usually go away within days or a few weeks. With permanent (seed) brachytherapy in the treatment of prostate cancer, there is a low probability that some seeds will migrate from the treatment region into the bladder or urethra and be released into the urine.
Brachytherapy for skin cancer can lead to symptoms similar to sunburn in the weeks after the treatment and thus to flaking of the upper layers of the skin around the treatment area. This usually heals in 5–8 weeks. If the cancer being treated is on the lip, an ulcer may develop as a result of brachytherapy, but this usually disappears after 4–6 weeks.
Most acute side effects associated with brachytherapy can be managed with medication or dietary measures and will resolve over time, usually within a few weeks of stopping treatment. The acute side effects of HDR brachytherapy are largely similar to those of external radiation therapy (EBRT).
Long term
In a small proportion of patients, brachytherapy could cause long-term side effects because neighboring tissue structures or organs have been damaged or disturbed. Long-term side effects are usually mild or moderate. For example, urinary and digestive problems as a result of brachytherapy for cervical or prostate cancer may persist and require further treatment.
Brachytherapy in the treatment of prostate cancer can cause erectile dysfunction (in approximately 15–30% of patients). However, the risk of erectile dysfunction is related to age (older men are at higher risk than younger ones) and the level of erectile function before starting brachytherapy. Most erectile dysfunction patients can be successfully treated with drugs such as Viagra . The risk of erectile dysfunction after treatment is lower with brachytherapy than with radical prostatectomy .
Brachytherapy for breast or skin cancer can cause scar tissue to form around the treatment area. With breast brachytherapy, fat necrosis can occur as a result of fatty acids that penetrate the breast tissue. This can mean that the breast tissue is swelling and tender. Fat necrosis is benign and occurs in about 2% of patients (usually 4–12 months after treatment).
Safety of people in the immediate vicinity
Patients who have been treated with brachytherapy often wonder whether certain safety precautions need to be taken against people in their immediate vicinity. With temporary brachytherapy, no radioactive sources remain in the body after the treatment. There is therefore no risk of radiation to friends or family when they are around.
In the case of permanent brachytherapy, weakly dosed radioactive sources (seeds) remain in the body after the treatment. The radiation dose rates are very low and completely disappear over time. The radiation is also only emitted to the tissue in the immediate vicinity (within a few millimeters of the tumor to be treated). As a precautionary measure, some patients on permanent brachytherapy may be advised not to have young children or to be too close to pregnant women immediately after treatment. Radiation oncologists and radiology assistants can give patients more information.
procedure
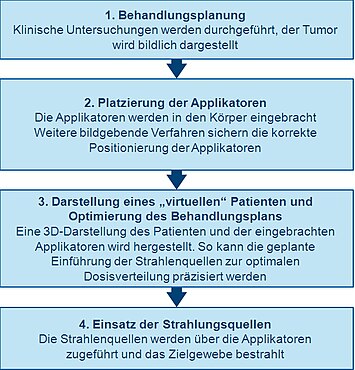
First planning
A thorough clinical examination is carried out for precise planning before performing brachytherapy in order to gain clarity about the specific characteristics of the tumor. In addition, a variety of imaging techniques can be used to visualize the shape and size of the tumor and its relationship to the surrounding tissues and organs. X-ray images, ultrasound examinations, computed tomography (CT or CAT) or magnetic resonance tomography (MRT) are used for this. With the aid of the examination data, three-dimensional visualizations of the tumor and the surrounding tissue can be obtained.
With this information, a plan for the optimal distribution of the radiation sources or nuclides can be developed. This includes considering how the applicators that are used to guide the radiation source into the treatment area should be placed and positioned. The applicators are not radioactive - usually needles or plastic catheters. The type of applicator used depends on the type of cancer being treated and the characteristics of the tumor. This initial planning helps to ensure that underdosing (“cold spot”) and overdosing (“hot spot”) are avoided during treatment. These could lead to treatment failure or side effects.
Introduction and illustration of the applicator (s)
Before radioactive substances or nuclides can be added, applicators (guides) must be introduced into the treatment area and correctly positioned in accordance with the initial planning.
Imaging techniques such as X-rays, fluoroscopy and ultrasound are commonly used to guide the applicator (s) to their correct position and to further develop the treatment plan. CT or MRT images can also be used. Once the applicators have been inserted, they are fixed in their correct position by means of sewing or adhesive tape to prevent a change in position. After it is certain that the applicators are correctly placed, further imaging procedures can be used to plan treatment.
Representation of a "virtual patient"
While the patient is in a separate, shielded treatment room, images of the patient (with the applicators used) are made and transferred to special software for treatment planning. The software enables the translation of two-dimensional images of the body region to be treated into a three-dimensional representation. The spatial relationships between the applicators, the treatment area and the surrounding healthy tissue in this "virtual patient" represent a copy of the relationships in the real patient.
Optimization of the treatment plan
In order to determine the optimal spatial and temporal distribution of the radiation sources within the applicators implanted in the tissue or cavity, virtual radiation sources are placed in the virtual patient using the special software for treatment planning. The software shows a graphic representation of the radiation distribution. In the run-up to the actual irradiation, this serves as an orientation aid for the treating physicians to specify the distribution of the radiation sources and to create a treatment plan that is optimally tailored to the anatomy of each individual patient. This procedure is sometimes referred to in English as "dose painting".
Use of radiation sources
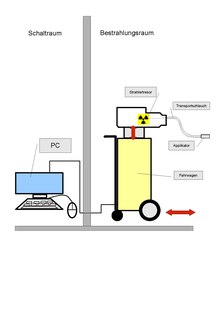
The radiation sources used for brachytherapy are always locked in a safe. The technique known as “afterloading” is common for interstitial and intracavitary brachytherapy.
"Afterloading" means that - after the precise placement of the non-radioactive applicators in the target volume (for example in the uterus ) - radiation sources (for example 137 cesium ) are supplied ("reloaded"). With manual reloading, the radiation source is inserted into the applicator by the doctor's hand. Due to the risk of radiation exposure of the hospital staff, manual feeding is limited to a few LDR applications.
Remote-controlled afterloading systems offer medical personnel protection from radiation exposure, as the radiation source is secured in a shielded safe. When the applicators are correctly positioned in the patient, a series of extension tubes connect them to the afterloading device that contains the radiation source. The treatment plan is sent to the afterloading device, which then monitors the introduction of the emitters through the extension hoses into the previously determined positions in the applicator. This process is only started after the staff has left the treatment room. The radiation sources remain in the respective position for a certain period of time in accordance with the treatment plan. They are then pulled back to the afterloading device via the extension hoses.
At the end of the brachytherapy treatment, the applicators are carefully removed from the body ( decorporation ). In general, this form of treatment does not pose a burden for the patient. Brachytherapy can usually be carried out on an outpatient basis.
Irradiation material
Electronic brachytherapy
In electronic brachytherapy, miniaturized low-energy X-ray tubes are placed in body / tumor cavities via a pre-positioned applicator in order to be able to deliver high doses quickly into the target tissue. In contrast, only low doses reach more distant healthy tissue.
Radionuclides
Radiation sources (radionuclides) frequently used for brachytherapy are (alphabetically):
| Radionuclide | Art | Half-life | energy |
|---|---|---|---|
| Cesium-137 ( 137 Cs) | γ radiation | 30.17 years | 0.662 MeV |
| Cobalt-60 ( 60 Co) | γ radiation | 5.26 years | 1.17; 1.33 MeV |
| Iridium-192 ( 192 Ir) | γ radiation | 73.8 days | 0.38 MeV (mean) |
| Iodine-125 ( 125 I) | γ radiation | 59.6 days | 27.4; 31.4 and 35.5 keV |
| Palladium-103 ( 103 Pd) | γ radiation | 17.0 days | 21 keV (medium) |
| Ruthenium-106 ( 106 Ru) | β radiation | 1.02 years | 3.54 MeV |
Web links
Individual evidence
- ↑ a b c d e Gerbaulet A et al .: Cervix carcinoma . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ a b c d e f g Ash D et al .: Prostate cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ a b c d Van Limbergen E et al .: Breast cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ a b c d e Van Limbergen E et al .: Skin cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ a b c d e f Gerbaulet A et al .: General aspects . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ a b Stewart AJ et al .: Radiobiological concepts for brachytherapy . In: Devlin P (Ed.): Brachytherapy. Applications and Techniques . LWW, Philadelphia 2007.
- ↑ a b c Viswanathan AN et al .: Gynecologic brachytherapy . In: Devlin P (Ed.): Brachytherapy. Applications and Techniques . LWW, Philadelphia 2007.
- ↑ a b Pickles T et al ..: Brachytherapy or Conformal External Radiotherapy for Prostate Cancer: A Single-Institution Matched-Pair Analysis . In: International Journal of Radiation Oncology - Biology - Physics . 2009.
- ↑ a b Haie-Meder C et al .: DVH parameters and outcome for patients with early-stage cervical cancer treated with preoperative MRI-based low dose rate brachytherapy followed by surgery . In: Radiotherapy and Oncology . 93, No. 2, 2009, p. 316.
- ↑ a b c Battermann J et al .: Results of permanent prostate brachytherapy, 13 years of experience at a single institution . In: Radiotherapy and Oncology . 71, No. 1, 2004, pp. 23-28.
- ↑ a b c Galalae R et al .: Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer . In: International Journal of Radiation Oncology . 58, 2004, pp. 1048-1055.
- ↑ a b c Hoskin PJ et al .: High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomized phase three trial . In: Radiotherapy and Oncology . 84, No. 2, 2007, pp. 114-120.
- ^ A b Pieters BR et al .: Comparison of three radiotherapy modalities on biochemical control and overall survival for the treatment of prostate cancer: A systematic review . In: Radiotherapy and Oncology . 93, No. 2, 2009, p. 168.
- ↑ a b c d Nelson JC et al .: Four-year clinical update from the American Society of Breast Surgeons MammoSite brachytherapy trial . In: The American Journal of Surgery . 198, 2009, pp. 83-91.
- ↑ Ferrer M et al .: Health-Related Quality of Life 2 Years After Treatment with Radical Prostatectomy, Prostate Brachytherapy, or External Beam Radiotherapy in Patients with Clinically Localized Prostate Cancer . In: International Journal of Radiation OncologyBiologyPhysics . 72, 2007, p. 421.
- ↑ a b c d e Frank S et al .: An Assessment of Quality of Life Following Radical Prostatectomy, High Dose External Beam Radiation Therapy and Brachytherapy Iodine Implantation as Monotherapies for Localized Prostate Cancer . In: The Journal of Urology . 177, No. 6, 2007, pp. 2151-2156.
- ↑ a b Gupta VK .: Brachytherapy - past, present and future . In: Journal of Medical Physics . 20, 1995, pp. 31-38.
- ↑ a b c d e Nag S: A brief history of brachytherapy . Retrieved September 25th, 2009.
- ^ Aronowitz J .: The “Golden Age” of prostate brachytherapy: A cautionary tale . In: Brachytherapy . 7, No. 1, 2008, pp. 55-59.
- ↑ Arne Grün, Thomas Kuhnt et al.: Re-irradiation for locally recurrent head and neck tumors and for prostate cancer. In: Deutsches Ärzteblatt. Volume 117, Issue 10, March 6, 2020, pp. 167–174, here: p. 169.
- ↑ a b c Thomadsen BR et al .: Brachytherapy Physics . Medical Physics Publishing, 2005.
- ↑ a b c d Mazaron JJ et al .: GEC-ESTRO recommendations for brachytherapy for head and neck squamous cell carcinomas . In: Radiotherapy and Oncology . 91, No. 2, 2009, pp. 150-156.
- ↑ a b Lartigau E et al .: Soft tissue sarcomas of the extremities in adults . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ a b Koukourakis G et al .: Brachytherapy for prostate cancer: A systematic review . In: Adv Urol . 2009. PMID 2735748 .
- ↑ a b Pötter R et al .: Oesophageal cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ a b Van Limbergen E et al .: Bronchus cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ a b c Nag S .: High dose rate brachytherapy: its clinical applications and treatment guidelines . In: Technology in Cancer Research and Treatment . 3, 2004, pp. 269-87. PMID 15161320 .
- ↑ a b Flynn A et al .: Isotopes and delivery systems for brachytherapy . In: Hoskin P, Coyle C (Eds.): Radiotherapy in practice: brachytherapy . Oxford University Press, New York 2005.
- ↑ a b Moule RN et al .: Non-surgical treatment of localized prostate cancer . In: Surgical Oncology . 18, No. 3, 2009, pp. 255-267.
- ↑ Mazeron JJ et al .: Brain cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ Pötter R et al .: Uveal melanoma . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ Dvorák et al .: Intraluminal high dose rate brachytherapy in the treatment of bile duct and gallbladder carcinomas . In: Hepato-gastroenterology . 49, No. 46, 2002, pp. 916-7.
- ↑ Ash D et al .: Bile duct cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ Mazeron JJ et al .: Anorectal cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ Van Limbergen E et al .: Urinary bladder cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ Gerbaulet A et al .: Urethral cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ^ Gerbaulet A et al .: Penis cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ Pötter R et al .: Endometrial cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ^ Gerbaulet A et al .: Primary vaginal cancer . In: Gerbaulet A, Pötter R, Mazeron J, Limbergen EV (eds.): The GEC ESTRO handbook of brachytherapy . ACCO, Belgium 2005.
- ↑ Joseph KJ et al .: Analysis of health related quality of life (HRQoL) of patients with clinically localized prostate cancer, one year after treatment with external beam radiotherapy (EBRT) alone versus EBRT and high dose rate brachytherapy (HDRBT) . In: Radiation Oncology . 3, No. 20, 2008.
- ↑ Holmboe P et al .: Treatment decisions for localized prostate cancer: asking men what's important . In: Journal of general internal medicine . 15, No. 10, 2000, pp. 694-701.
- ↑ Hoskin P, Coyle C (Ed.): Radiotherapy in practice: brachytherapy . Oxford University Press, New York 2005.
- ↑ Guedea F et al .: Patterns of Care for Brachytherapy in Europe: Facilities and resources in brachytherapy in the European area . In: Brachytherapy . 7, No. 3, 2000, pp. 223-230.
- ↑ Quang TS et al .: Technological evolution in the treatment of prostate cancer . In: Oncology . 21, 2007.
- ↑ a b Guedea F et al .: Quality of life two years after radical prostatectomy, prostate brachytherapy or external beam radiotherapy for clinically localized prostate cancer: the Catalan Institute of Oncology / Bellvitge Hospital experience . In: Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico . 11, No. 7, 2007, pp. 470-8.
- ^ Litwin et al .: Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer . In: Cancer . 109, No. 11, 2007, pp. 2239-2247.
- ↑ Pistis F et al .: External beam radiotherapy plus high-dose-rate brachytherapy for treatment of locally advanced prostate cancer: the initial experience of the Catalan Institute of Oncology . In: Brachytherapy . 9, No. 15, 2009.
- ↑ a b Lertsanguansinchai P et al .: Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma . In: International Journal of Radiation OncologyBiologyPhysics . 59, 2009, p. 1424.
- ^ Gaffney P et al .: Practice Patterns of Radiotherapy in Cervical Cancer Among Member Groups of the Gynecologic Cancer Intergroup (GCIG) . In: International Journal of Radiation OncologyBiologyPhysics . 68, 2007, pp. 485-490.
- ↑ a b National Institute for Health and Clinical Excellence: High dose rate brachytherapy for carcinoma of the cervix . NICE. March 2006. Retrieved September 25th, 2009.
- ↑ a b c Viswanathan AN et al .: American Brachytherapy Society cervical cancer brachytherapy task group (PDF; 45 kB) American Brachytherapy Society. Retrieved September 25th, 2009.
- ↑ Viswanathan AN et al .: Three-Dimensional Imaging in Gynecologic Brachytherapy: A Survey of the American Brachytherapy Society . In: International Journal of Radiation OncologyBiologyPhysics . 2009.
- ↑ Kim et al .: High-Dose Rate Brachytherapy Using Inverse Planning Simulated Annealing for Locoregionally Advanced Cervical Cancer: A Clinical Report with 2-Year Follow-Up . In: International Journal of Radiation OncologyBiologyPhysics . 75, 2009, p. 1329.
- ↑ Pötter et al .: Present status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma . In: Acta Oncologica . 47, No. 7, 2008, pp. 1325-1336.
- ↑ Pötter et al .: Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy - 3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics , radiobiology . In: Radiotherapy and Oncology . 78, No. 1, 2006, pp. 67-77.
- ↑ Hareyama SK et al .: High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix: a randomized trial . In: Cancer . 94, No. 1, 2008, pp. 117-24.
- ↑ a b Merrick GS et al .: American Brachytherapy Society prostate low-dose rate task group (PDF; 174 kB) American Brachytherapy Society. Retrieved September 25th, 2009.
- ↑ a b Hsu IC et al .: American Brachytherapy Society prostate high-dose rate task group (PDF; 97 kB) American Brachytherapy Society. Retrieved September 25th, 2009.
- ↑ a b Ash D et al .: Prostate Cancer . In: Hoskin P, Coyle C (Eds.): Radiotherapy in practice: brachytherapy . Oxford University Press, New York 2005.
- ^ Morris WJ et al .: Evaluation of Dosimetric Parameters and Disease Response After 125Iodine Transperineal Brachytherapy for Low- and Intermediate-Risk Prostate Cancer . In: International Journal of Radiation OncologyBiologyPhysics . 73, 2009, pp. 1432-1438.
- ↑ a b Prostate cancer: internal radiotherapy (brachytherapy) ( Memento from April 4, 2009 in the Internet Archive )
- ↑ a b Pisansky et al .: High-dose-rate brachytherapy in the curative treatment of patients with localized prostate cancer . In: Mayo Clinic proceedings. Mayo Clinic . 83, No. 12, 2008.
- ↑ Pistis et al .: External beam radiotherapy plus high-dose-rate brachytherapy for treatment of locally advanced prostate cancer: the initial experience of the Catalan Institute of Oncology . In: Brachytherapy . 9, 2009, p. 15.
- ↑ a b c Keisch et al .: American Brachytherapy Society breast brachytherapy task group (PDF; 97 kB) American Brachytherapy Society. February 2007. Retrieved September 25th, 2009.
- ↑ a b Hoskin P et al .: Breast Brachytherapy . In: Hoskin P, Coyle C (Eds.): Radiotherapy in practice: brachytherapy . Oxford University Press, New York 2005.
- ↑ a b Polgár C et al .: Current status and perspectives of brachytherapy for breast cancer . In: International Journal of Clinical Oncology . 14, 2009, p. 7.
- ↑ Kelley JR et al .: Breast brachytherapy . In: Devlin P. (Ed.): Brachytherapy. Applications and Techniques . LWW, Philadelphia 2007.
- ↑ King et al .: Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T (is, 1,2) breast cancer . In: American journal of surgery . 180, No. 4, 2000, pp. 299-304.
- ↑ Go'mez-Iturriaga A et al .: Early breast cancer treated with conservative surgery, adjuvant chemotherapy, and delayed accelerated partial breast irradiation with high-dose-rate brachytherapy . In: Brachytherapy . 7, No. 4, 2008, pp. 310-315.
- ↑ Brachytherapy patient guide (PDF; 770 kB)
- ↑ Guix et al .: Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds . In: International journal of radiation oncology, biology, physics . 47, No. 1, 2000, pp. 95-102.
- ↑ Sedda AF et al .: Dermatological high-dose-rate brachytherapy for the treatment of basal and squamous cell carcinoma . In: Clinical and Experimental Dermatology . 33, No. 6, 2008, pp. 745-749.
- ↑ Rio E et al .: Interstitial brachytherapy of periorificial skin carcinomas of the face: A retrospective study of 97 cases . In: International Journal of Radiation OncologyBiologyPhysics . 63, 2005, pp. 753-757.
- ↑ a b Musmacher J et al .: High dose rate brachytherapy with surface applicators: Treatment for nonmelanomatous skin cancer . In: Journal of Clinical Oncology . 24, 2006, p. 15543.
- ↑ Members A et al .: Guidelines for Percutaneous Coronary Interventions: the Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology . In: European Heart Journal . 26, No. 8, August, p. 804.
- ↑ Sidawy et al .: Peripheral vascular brachytherapy . In: Journal of Vascular Surgery . 35, No. 5, August, pp. 1041-7.
- ↑ Pérez-Castellano N et al .: Pathological Effects of Pulmonary Vein beta-Radiation in a Swine Model . In: Journal of Cardiovascular Electrophysiology . 17, No. 6, August, pp. 662-669.
- ↑ Macmillan Cancer Support: Brachytherapy ( Memento from September 17, 2013 in the Internet Archive )
- ↑ a b Fieler .: Side effects and quality of life in patients receiving high-dose rate brachytherapy . In: Oncology nursing forum . 24, No. 3, August, pp. 545-53.
- ↑ a b c d Doust et al .: A systematic review of brachytherapy. Is it an effective and safe treatment for localized prostate cancer? . In: Australian family physician . 33, No. 7, August, pp. 525-9.
- ↑ a b c Magné N et al .: Patterns of care and outcome in elderly cervical cancer patients: A special focus on brachytherapy . In: Radiotherapy and Oncology . 91, No. 2, August, pp. 197-201.
- ^ Casino AR et al .: Brachytherapy in lip cancer . In: Medicina Oral . 11, 2006, pp. E223-9.
- ↑ Vicini F et al .: Three-year analysis of treatment efficacy, cosmesis, and toxicity by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation (APBI) . In: Cancer . 112, No. 4, 2008, pp. 758-766.
- ↑ Brachytherapy patient guide (PDF; 277 kB)
literature
- Bavarian State Ministry for the Environment, Health and Consumer Protection: Radioactivity, X-rays and Health. 2006.
- Alain Gerbaulet et al. (Ed.): The GEC / ESTRO Handbook of Brachytherapy ( Memento of April 20, 2015 in the Internet Archive ) (English) ACCO, Leuven, Belgium, 2002. ISBN 90-804532-6
- Hanno Krieger: Radiation sources for technology and medicine. Teubner, Wiesbaden, 2005, ISBN 3-8351-0019-X .
- Hanno Krieger: Fundamentals of radiation physics and radiation protection. Vieweg + Teubner, Wiesbaden, 3rd edition 2009, ISBN 978-3-8348-0801-1 .
- Michael Wannenmacher, Jürgen Debus, Frederik Wenz (Eds.): Radiotherapy. Springer, Berlin Heidelberg, 2006, ISBN 3-540-22812-8 .
- Edward C. Halperin, Carlos A. Perez, Luther W. Brady: Perez and Brady's Principles and Practice of Radiation Oncology. Lippincott Williams & Wilkins, 2008, ISBN 978-0-7817-6369-1 , p. 483. ( books.google.com ).