Chagas disease
| Classification according to ICD-10 | |
|---|---|
| B57 | Chagas disease |
| ICD-10 online (WHO version 2019) | |
The Chagas disease [ ʃaːgas- ] (also called American trypanosomiasis , South American trypanosomiasis or Chagas disease hereinafter) is an infectious disease and parasitosis caused by the protozoa Trypanosoma cruzi is caused. It is transmitted through the feces of predatory bugs . Its clinical picture is characterized by facial edema and swelling of the lymph nodes
distribution
It is mainly found in Central and South America and is transmitted through the droppings of blood-sucking predatory bugs . The predatory bugs also infect each other through coprophagia and "cannibalism". A pathogen reservoir (see also zoonosis ) consists of a. in wild animals (e.g. armadillos , possums , two-toed sloths ), but also in dogs, cats and rats. Infected humans are also an important reservoir for parasites. In total, there are said to be more than 18 million infected people. Around a quarter of the population in Bolivia could be affected. There are 50,000 new infections and 15,000 deaths annually.
Migration, tourism and bioinvasion of the predatory bugs through the transport of goods can bring the disease to other continents and, in rare cases, spread it there through blood donations. In Spain, where over 200,000 immigrants from Latin America live, there are an estimated 6,000 infected people. Several hundred thousand immigrants could be infected in the United States. There are three confirmed cases of Chagas disease caused by blood products; in 2006, one of 4655 blood products checked was positive in routine tests.
In May 2012, Uruguay announced the extermination of the transmitted predatory bug , known in South America as Vinchuca .
Pathogen and vector
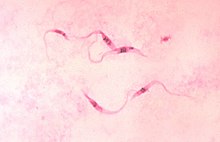
The pathogen Trypanosoma cruzi is a unicellular organism that is transmitted by predatory bugs (Reduviidae). The vectors ( vector ) are three to four centimeters large predatory bugs of the genera Triatoma , Rhodnius and Panstrongylus (all from the subfamily Triatominae ), with Triatoma infestans being the most important insect host. All stages, including the larvae, are susceptible to Trypanosoma cruzi .
Transmission of T. cruzi from bed bugs to mice and from mice to bed bugs has also been demonstrated in laboratory experiments . To what extent bed bugs can play a role in the transmission of Chagas disease to humans is unclear; a systematic analysis of bed bug populations in regions with T. cruzi is still pending .
Routes of infection
The mostly nocturnal predatory bugs sting and sucking blood mostly unnoticed in sleeping humans, mammals, reptiles and birds, with preference in regions with thinner skin like z. B. on lips or in the eye area. Meanwhile defecated the insect. The infection does not occur through the sting, but rather through rubbing the pathogen-containing excrement into the fresh stab wound by the person himself or through penetration of the pathogen into uninjured mucous membrane, especially the eye. The feces can probably remain infectious for years. The placental infection of the fetus through the mother is possible. Breast milk is also infectious. In rare cases, it can be transmitted through food. In 2005 and 2006, infections from contaminated Bacaba wine (from palm fruits, Oenacarpus distichus , Oenocarpus babaca ) were reported from Brazil , and the juice of the cabbage palm ( Euterpe oleracea ) is also suspected.
Course / symptoms
Chagas disease occurs in humans in four stages: After the bite of the robber bug, swelling usually occurs around the stab wound created by the insect: An edema with symptoms of inflammation, often near the eyes ( Romana sign or Romanasque sign ). However, other parts of the body can also be affected that may not be identifiable later.
An acute phase occurs in about a third of the newly infected. They are mostly children or people with a weak immune system. This phase usually subsides after about four weeks. Fever, shortness of breath, edema , diarrhea, abdominal pain, swelling of the lymph nodes, seizures when the brain is included and enlarged heart are the result.
After a latency phase, which u. Chronic illness can occur. Symptoms of the acute phase can also break out again during the latency phase if a newly emerging immune system is added, e.g. B. HIV infection.
The symptoms of the chronic phase arise mainly from the chronic enlargement of the heart (palpitations, poor performance, shortness of breath during exercise) and the destruction of nerve cells in the digestive tract. This leads to massive swelling, especially of the esophagus (megaesophagus) and colon (megacolon). As a result, normal intestinal passage is no longer possible. This can lead to death from a ruptured bowel , an obstruction of the bowel (ileus) or inflammation of the peritoneum (peritonitis).
If left untreated, Chagas disease can be fatal in up to 10% of cases. Infants and young children are particularly at risk.
diagnosis
The pathogen can be detected microscopically in the blood ( blood smear or thick drops ) , especially in the acute phase in the first few weeks . In the chronic phase of the disease, the pathogen is detected with antibody tests (e.g. immunofluorescence ). In South America there is the trypanosome test in the form of the so-called xenodiagnosis . For this purpose, lab-grown bugs that are free of pathogens are allowed to take a blood meal on the patient's skin. After two to four weeks (according to different sources) the intestines of the predatory bugs are examined for infestation. The effects of the disease can be demonstrated in the brain using CT or MRI . Echocardiography can be used to examine the heart .
An ELISA -based, FDA- approved blood test has been available in the USA since 2007 .
prevention
There is currently no approved trypanosomiasis vaccine. The predatory bugs are fought to prevent the disease. They like to live near the sleeping places of the pets, so those places are to be segregated. Sufficiently closed apartments as well as tent-like mosquito nets that are also completely closed at the bottom and with a tight zipper offer very good protection, provided that the net is not touched while sleeping. If necessary, the conventional mosquito net must be tucked under the mattress. The most endangered sleeping places are in open, simple houses z. B. with walls and roofs made of straw and similar wattle. Many unspecific insecticides or repellants are usually ineffective against predatory bugs .
Continuous monitoring of blood donations is intended to prevent the possibility of the infection being transmitted during blood transfusions and transplants . However, this does not always happen reliably in the affected countries.
A team of researchers led by parasitologist and infection biologist Sven Klimpel found out in 2020 that (partly as a result of global warming ) the bugs could also make their home in Portugal, France, Spain, Italy, Central Africa and Southeast Asia. The authors of the study recommend making the infection notifiable .
therapy
Drug therapy is difficult. The only available drugs, nifurtimox and benznidazole , are mainly effective in the acute phase of the disease, sometimes have severe side effects and are considered mutagenic . In addition, some pathogens are resistant to the agents. The more difficult-to-treat chronic forms are to be avoided through better control of diseases in children. According to WHO statistics, however, the number of new infections has decreased significantly due to the control of the insect. Due to the wide variety of distribution maps that are offered on the Internet, as a precautionary measure, appropriate prophylaxis should be taken in the entire distribution area of the pathogen carrier; this could also include areas in the USA.
history
The disease is named after the Brazilian doctor and infectious disease specialist Carlos Chagas , who discovered the pathogen in 1907 and first described the disease in 1909. Until the 1960s, however, the disease was not considered a major epidemiological problem. Chagas recognized flagellates in the intestines of Triatominae and proved that they can be transmitted to monkeys by the bug sting. Chagas named the parasite after Oswaldo Cruz , a well-known Brazilian doctor and epidemiologist, after whom the institute he worked at is named.
What is outstanding about Chagas' work is that he alone described the complete mechanism of the disease: the causative pathogen , the vector , the host , the clinical manifestation and the epidemiology.
For a long time, Chagas mistook the sting for the main source of infection:
“Through irrefutable experiments [...] it has been proven that the triatomic sting is the natural mechanism for the transmission of the trypanosome. Brumpt claims that transmission takes place primarily through stool deposits in the skin and mucous membrane of humans, but I believe that this certainly possible process is an exception: the insect bite is the biological infection process of this trypanosome. "
In 1969, the British parasitologist Ralph Lainson was the first to describe the disease correctly. He discovered that food contaminated by blood-sucking triatominae transmitted the disease.
literature
- E. Sourdough, T. Weinke: American Trypanosomiasis (Chagas Disease). In: W. Lang, T. Löscher (Ed.): Tropical medicine in clinic and practice. 3rd edition, Thieme, Stuttgart 2000, pp. 59-67.
- Louis V. Kirchhoff: American Trypanosomiasis (Chagas's Disease). In: Richard L. Guerrant, David H. Walker, Peter F. Weller (Eds.): Tropical Infectious Diseases: Principles, Pathogens and Practice. 2nd Edition. Churchill Livingstone, 2005. pp. 1082-1094.
See also
Web links
- Info from the Tropical Institute Hamburg
- Latest news on the spread of Chagas disease Information from the WHO
- International research initiative Chagaspace ( Memento of April 7, 2008 in the Internet Archive )
Individual evidence
- ↑ wired.com, May 30, 2012, Chagas Disease: Poverty, Immigration, and the 'New HIV / AIDS'
- ↑ Chagas disease: a neglected emergency. In: Lancet. Volume 373, Number 9678, May 2009, pp. 1820, ISSN 1474-547X . doi : 10.1016 / S0140-6736 (09) 61002-3 . PMID 19482198 .
- ↑ GA Schmunis: Epidemiology of Chagas disease in non-endemic countries: the role of international migration. In: Memórias do Instituto Oswaldo Cruz. Volume 102 Suppl 1, October 2007, pp. 75-85, ISSN 0074-0276 . PMID 17891282 .
- ↑ CDC: Blood donor screening for chagas disease - United States, 2006-2007. In: MMWR. Morbidity and mortality weekly report. Volume 56, Number 7, February 2007, pp. 141-143, ISSN 1545-861X . PMID 17318113 .
- ↑ Martín Cajal: Uruguay sin mal de chagas , El Diario , May 25, 2012.
- ↑ Salazar et al. (2014) Bed bugs ( Cimex lectularis ) as vectors of Trypanosoma cruzi . Am J Trop Med Hyg. Pii: 14-0483 doi : 10.4269 / ajtmh.14-0483 . PMID 25404068 .
- ↑ elifesciences.org May 6, 2020: Modeling the climatic suitability of Chagas disease vectors on a global scale
- ^ Wolfgang U. Eckart : Chagas disease. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 235 f., Here: p. 235.
- ↑ Carlos Chagas: American Trypanosomiasis ( Chagas' disease): Brief aetiological and clinical considerations . s. l., sn, 1925. 14 p. - online ( Memento from July 9, 2007 in the Internet Archive ) (PDF; 49 kB)
- ↑ Ralph Lainson obituary in: The Guardian May 18, 2015, accessed May 18, 2015.