Hydrogen electrode
The term hydrogen electrode is generally used for electrodes on which hydrogen gas H 2 is evolved or consumed. The hydrogen electrode is an important aid for measurements in electrochemistry and physical chemistry : It is one of the most important reference electrodes , which means that it is used to determine the potential of other electrodes by means of a simple voltage measurement . The potential is the most important variable for describing the electrochemical state of an electrode.
The normal (NHE) and standard hydrogen electrodes (SHE) are particularly important, as their potential is defined as the zero point on the standard potential scale. The potential of hydrogen electrodes is given by the reaction:
described.
Standard hydrogen electrode (SHE)
The standard hydrogen electrode ( engl. Standard Hydrogen Electrode , short SHE) is an ion activity a hydrogen pressure of 1013 of 1 mol / l and hPa normalized at each temperature. Like every reference electrode, it serves the purpose of delivering a precisely defined potential. In addition, it is particularly suitable for determining standard potentials, since its potential is by definition the zero point on the potential scale. The absolute electrode potential of the hydrogen electrode is at 298.15 K to IUPAC 4.44 ± 0.02 recommendation V .
In contrast to the standard hydrogen electrode, the normal hydrogen electrode (NHE = Normal Hydrogen Electrode) works with the ion concentration of hydrogen under otherwise identical conditions.
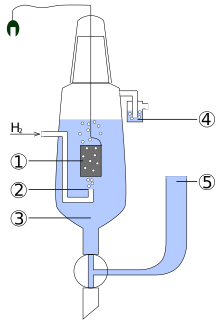
Layout and function
The potential of the hydrogen electrode is based on the following reaction:
The technical problem with a hydrogen electrode is that hydrogen as a gas can neither take the form of an electrode nor conduct electricity. An auxiliary electrode in the form of a neutral precious metal (platinum) is therefore used. This is flushed with hydrogen gas, which adsorbs on the platinum surface and covers it with a wafer-thin layer of hydrogen atoms. This results in a "hydrogen block" made of platinum inside - the hydrogen electrode.
The standard hydrogen electrode, which defines the zero point of the standard potential scale, therefore, consists of a platinum-plated (i.e., electrolytically.. Platinum black coated) platinum foil, which in an acid - solution with an ion activity of the protons of dips. The platinum electrode is of hydrogen gas at a pressure of 1013 hPa and a temperature of 298.15 K lapped. The hydrogen gas partially adsorbs on the platinum , forms an equilibrium with the hydrogen ions in the acid and thus determines the electrochemical potential .
The electrical potential that forms on this electrode is the standard potential , which is defined as being identical to zero .
Importance and handling
Since standard potentials are measured relative to the standard hydrogen electrode, this electrode is of great importance for physical chemistry . However, they are very cumbersome to handle: On the one hand, they require handling of hydrogen gas , whereby the formation of oxyhydrogen gas must be avoided by using closed vessels . An oxygen-free atmosphere in the electrolyte vessel is also necessary because oxygen can distort the potential of the electrode. On the other hand, a platinum electrode is prone to contamination, so no traces of grease, e.g. B. of fingerprints; to ensure high catalytic activity, it should be re-platinized. Pressure control is also required for precise measurements. For these reasons, hydrogen electrodes are rarely used for routine examinations, just as rarely for research work, unless these are aimed at determining standard potentials. Calomel or silver-silver chloride electrodes (Ag / AgCl), in which no gaseous reactants occur, so that practically no pressure dependence occurs , were used much more frequently . Today Ag / AgCl electrodes are mainly used to avoid the poisonous calomel. In contrast to hydrogen electrodes, they are also commercially available in many different ready-to-measure variants.
Normal hydrogen electrode (NHE)
The standard conditions of the SHE cannot be set in the experiment. This includes the activity of the protons and the gas pressure of 1013 hPa. It is therefore recommended to use 1 mol / l hydrochloric acid and hydrogen gas as the electrolyte under atmospheric conditions. In this case one speaks of the normal hydrogen electrode. The deviations from the SHE are minimal, but are pH-dependent due to the Nernst equation .
Reversible hydrogen electrode (RHE)
→ Main article: Reversible hydrogen electrode
For some electrochemical investigations, hydrogen electrodes are also used, which are operated at ambient pressure; the hydrogen electrodes for water electrolysis often work at overpressure. For tests in acids or alkalis , a hydrogen electrode in the same solution as the electrode to be measured is sometimes used; it is not separated by a salt bridge . In this way it can be achieved that the measured potential does not change with the pH value .
Overvoltages occur during the electrolysis of water : the required cell voltage is higher than the equilibrium voltage due to kinetic inhibition. The overvoltage increases with increasing current density at the electrodes. The measurement of equilibrium potentials is therefore carried out without current if possible.
See also
- Electrode potential
- Reference electrode
- Electromotive force
- Salt bridge
- Redox potential
- Electrochemical series
Web links
Individual evidence
- ↑ Entry on standard hydrogen electrode . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.S05917 .


![a {\ mathrm {[H ^ {+}]}} = 1](https://wikimedia.org/api/rest_v1/media/math/render/svg/e1cb1f9d90be1cd090b9525b563116abbd977c26)