Ortho and para hydrogen
Ortho and para hydrogen (short o H 2 and p H 2 ) are two forms of the di hydrogen molecule that differ in the symmetry of their nuclear spin configuration and rotational states. Although they have the same chemical structure , some of them have different physical properties. Hydrogen is generally a mixture of both species; the equilibrium mixing ratio is a function of the temperature . The existence of the two forms must e.g. B. be taken into account in the liquefaction of hydrogen, especially if this is to be used as rocket fuel . In addition, the symmetry order of para hydrogen can be used for signal amplification of nuclear magnetic resonance ( hyperpolarization ), for nuclear magnetic resonance spectroscopy (NMR) and magnetic resonance tomography (MRT).
Physical basics
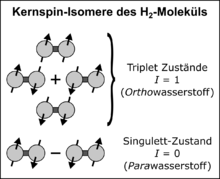
Since hydrogen nuclei are spin 1/2 particles, they follow the Fermi-Dirac statistics . Therefore, the quantum mechanical state of the entire hydrogen molecule (H 2 ) must be antisymmetric with regard to an exchange of the two protons ( Pauli principle ). For this, the total wave function of the two nuclei must be antisymmetric. This can be represented as the product of the spatial (orbital angular momentum) and spin wave function of the two protons of the H 2 molecule and is precisely antisymmetric if one of the two factors of the product is antisymmetric and the other is symmetric.
After adding the angular momentum of the nuclear spin, there are four eigenstates of the nuclear spin wave function: three triplet states with total spin and magnetic spin quantum numbers and a singlet state with and . The three triplet states are symmetrical and are called Ortho states ( ortho hydrogen, or o H 2 for short ); the singlet state is antisymmetric and is also called the Para state ( para hydrogen, p H 2 for short ). In Dirac notation , the four eigenstates are written as follows:
Since the total wave function is antisymmetric when the two protons are interchanged, the spatial wave function to the orbital angular momentum must be antisymmetric ( odd) in the triplet or ortho state (symmetric with respect to the nuclear spin of the protons) and antisymmetric ( odd) in the singlet or para state (antisymmetric with respect to the nuclear spin) symmetrical ( straight).
In general, hydrogen is a mixture of o H 2 and p H 2 (or all four nuclear spin eigenstates); the temperature-dependent equilibrium distribution satisfies the Boltzmann statistics . Since the energy split between the nuclear spin states is negligibly small compared to the rotational energies, the latter effectively decide on the energetic ground state of the H 2 molecule. Therefore, p H 2 is the energetically more favorable and o H 2 the more energetic form. The temperature-dependent equilibrium ratio of o H 2 / p H 2 is described by
- ,
where is the rotation temperature ( , with the rotation constant ). The limit value for high temperatures is and corresponds to a uniform distribution of the four nuclear spin states (75% o H 2 and 25% p H 2 ). This corresponds exactly to the degeneracy ratio of the nuclear spin states ( Ortho with total spin 1 three states, Para with total spin 0 not degenerate, that is, only one state). This ratio is almost reached at room temperature. For low temperatures ( ) , the limit value 0 corresponds to 100% p H 2 .
The transitions between o H 2 and p H 2 are extremely rare at normal pressure and not too high temperature in the gas phase due to the maintenance of symmetry (pure nuclear spin transitions are even prohibited) and the conversion, i.e. the approach to the equilibrium distribution, proceeds correspondingly slowly . However, this process can be accelerated with homogeneous as well as heterogeneous catalysis , for example with activated carbon .
Physical Properties
The behavior of the specific heat of hydrogen, especially at low temperatures, remained unexplained for a long time. In 1912 Arnold Eucken had observed the decrease in the rotational component of the specific heat in gaseous hydrogen between 300 and 60 Kelvin, which at the time could not be theoretically explained. Likewise had Reinhard Meckenheim 1922 an intensity change in the ratio 1: 3 discovered in the band spectra of diatomic molecules with identical atoms. Only after the development of quantum mechanics was Werner Heisenberg able to show in 1926 that the two spin isomers of the H 2 molecule are the cause of this, and he proceeded in analogy to the electrons in ortho- and para-helium. Heisenberg was later awarded the Nobel Prize in Physics “for the creation of quantum mechanics, the application of which, among other things, led to the discovery of the various forms of hydrogen” . Ortho and para hydrogen differ in some basic physical properties . They have significantly different heat capacities, but also different electrical conductivities and different temperature dependencies of these two quantities. In addition, o H 2 can be measured using the nuclear magnetic resonance (NMR) method, while p H 2 is “NMR-invisible” with total spin . Apart from that, the physical properties are only slightly different. For example, the melting and boiling points of the para form are about 0.1 K below those of the ortho form.
Applications
Production and storage of liquid hydrogen
Since the states of para hydrogen are energetically more favorable than those of ortho hydrogen, the energy difference is released in the form of heat during the ortho-para conversion . As mentioned above, however, the conversion is slow, which means that it takes place with a time delay during cooling and liquefaction (≈ 21 K) under normal conditions. The energy released (enthalpy of transformation) is 1544 J / mol, greater than the heat of vaporization (904 J / mol), which is necessary to convert liquid hydrogen into a gaseous state. Therefore, due to the ortho-para conversion, even with complete thermal insulation, a significant part of the liquid hydrogen will evaporate if appropriate precautions are not taken during liquefaction. In the industrial production of liquid hydrogen, the conversion during cooling is therefore accelerated catalytically in the gas phase .
Rocket propulsion
Liquid hydrogen is used as rocket fuel in hydrogen-oxygen-based rocket technologies, which means that larger amounts of H 2 can be carried. Here, too, accelerated evaporation through the o H 2 - p H 2 conversion would be undesirable, which is why liquid hydrogen is refueled in thermal equilibrium .
Parahydrogen in NMR and MRI
Enriched parahydrogen is used in chemistry and medicine to amplify the nuclear magnetic resonance signal (measured variable of NMR and MRT ) by several orders of magnitude ( hyperpolarization ). The pure state is used to transfer the spin order to a target molecule. The effect was discovered by Bowers and Weitekamp and called "PASADENA" (Parahydrogen And Synthesis Allow Dramatically Enhanced Nuclear Alignment). Today the method is often referred to as "PHIP" (ParaHydrogen Induced Polarization). The method was used to study the course of hydrogenation reactions. Current research is aimed at the development of new types of MRI contrast media .
Ortho and para water
As with the H 2 molecule, one can also distinguish between an ortho and a para state for the water molecule (H 2 O), depending on whether the spins of the hydrogen nuclei are parallel or antiparallel. (Except in the case of the very rare isotope 17 O, the oxygen nucleus has no spin.) The rotational states of the molecule are linked to the nuclear spin through quantum mechanical effects: the ground state of para-water has the rotational quantum number , that of ortho-water has the rotational quantum number . Para- and ortho-water could be separated with the help of strong electric fields and it could be proven that the two forms of water have different chemical properties: The reaction with the diazenylium ion
runs faster with para-water than with ortho-water.
See also
Helium atom # Ortho- and parahelium with analogous states of the electron spins in the helium atom
Individual evidence
- ^ Clifford R. Bowers: Sensitivity Enhancement Utilizing Parahydrogen . In: eMagRes . John Wiley & Sons, Ltd., New York 2007, ISBN 978-0-470-03459-0 , doi : 10.1002 / 9780470034590.emrstm0489 .
- ↑ a b Johannes Natterer, Joachim Bargon: Parahydrogen induced polarization . In: Progress in Nuclear Magnetic Resonance Spectroscopy . tape 31 , no. 4 , November 1997, ISSN 0079-6565 , p. 293-315 , doi : 10.1016 / S0079-6565 (97) 00007-1 .
- ↑ a b c The discovery of para-hydrogen. In: mpibpc.mpg.de. Max Planck Institute for Biophysical Chemistry .
- ↑ Important Scientists. Werner Heisenberg (1901–1976). In: physicsoftheuniverse.com. Retrieved August 16, 2016 .
- ^ Manne Siegbahn: The Physics Prize. In: Nobel Foundation: Nobel: The Man and His Prizes. (New York: Elsevier Publishing Co., 1962), pp. 492-497.
- ↑ Adalbert Farkas: Ortho Hydrogen, and heavy hydrogen parahydrogen . University Press, Cambridge 1935, DNB 362436835 .
- ^ J. Hord et al .: Selected Properties of Hydrogen (Engineering Design Data). ( Memento from February 20, 2017 in the Internet Archive ). (PDF; 16.3 MB).
- ↑ a b N. Getoff: hydrogen as a fuel: the production, storage, transportation. Springer-Verlag, 2013, ISBN 978-3-7091-7694-8 .
- ^ AH Larsen, FE Simon, CA Swenson: The Rate of Evaporation of Liquid Hydrogen Due to the Ortho ‐ Para Hydrogen Conversion. In: Rev. Sci. Instrum. 19: 266 (1948); doi: 10.1063 / 1.1741241 .
- ↑ J.-B. Hövener et al .: A continuous-flow, high-throughput, high-pressure parahydrogen converter for hyperpolarization in a clinical setting. In: NMR in Biomedicine. February 2013, Volume 26, No. 2, pp. 124-131. doi: 10.1002 / nbm.2827 .
- ^ C. Russell Bowers, Daniel P. Weitekamp: Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance . In: Physical Review Letters . tape 57 , no. November 21 , 1986, pp. 2645-2648 , doi : 10.1103 / PhysRevLett.57.2645 .
- ↑ Sharp images with the earth's magnetic field. Archived from the original on August 14, 2016 ; accessed on August 14, 2016 .
- ^ Daniel A. Horke, Yuan-Pin Chang, Karol Długołecki and Jochen Küpper: Separation of para and ortho water. In: Angewandte Chemie. 2015 , 126, pp. 12159–12162, doi : 10.1002 / anie.201405986 .
- ↑ Ardita Kilaj, Hong Gao, Daniel Rösch, Uxia Rivero, Jochen Küpper & Stefan Willitsch: Observation of different reactivities of para and ortho-water towards trapped diazenylium ions. In: Nature Communications. 2018 , 9, p. 2096, doi : 10.1038 / s41467-018-04483-3 .