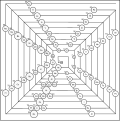
Periodic table
The periodic table (long version periodic table of the elements , abbreviated PSE or PSdE ) is a list of all chemical elements , arranged according to increasing nuclear charge ( atomic number ). The list is divided into rows (periods) so that each column (group) of the resulting table contains elements with similar chemical properties . The name periodic system (from Greek περίοδος períodos , German 'handling, circulation, cycle' ) indicates that many properties of the elements repeat themselves periodically with increasing atomic number.
The periodic table was presented in 1869 independently and almost identically by two chemists, first by the Russian Dmitri Mendeleev (1834–1907) and a few months later by the German Lothar Meyer (1830–1895). Historically, the periodic table was of particular importance for predicting as yet undiscovered elements and their properties, since the properties of an element can be approximately predicted if the properties of the surrounding elements in the periodic table are known. Today it is primarily used as a clear organization scheme for the elements and to determine possible chemical reactions .
Periodic table
Basic principle
A periodic table is a systematic tabular compilation of the chemical elements in which the elements are arranged according to two principles: On the one hand, they are arranged according to increasing atomic number (i.e. the number of protons in the atomic nucleus that is unique and characteristic for each element ). On the other hand, the representation is chosen so that elements with similar chemical behavior are close together. With increasing atomic number, the properties of the elements resemble each other in regular, albeit differently long, periodic intervals. The term "periodic table" indicates that these periodicities are represented by the selected arrangement of the elements.
presentation
There are different types of periodic tables. The best-known representation arranges the elements in a two-dimensional tabular grid, taking into account the periodicities, in which a grid box corresponds to each element. The horizontal lines of the display are called periods , the vertical columns are called groups .
Within each period the atomic number of the elements increases from left to right. The line breaks are chosen so that chemically similar elements are each in the same column (group). The elements of a group therefore show similar chemical behavior. For example, there is the group of chemically inert noble gases or the group of reactive halogens .
The periods have different lengths. The first period comprises only two elements. This is followed by two periods with eight elements each, two further periods with eighteen elements each and finally two periods with thirty-two elements each.
The long form of the periodic table, in which the last two periods are displayed as continuous lines, is often unfavorable because of the required width of the display. In the mostly used medium-length form, groups of elements cut out of these periods are shown below the main system to save space. In this form the periodic table has seven periods and eighteen groups. There is also an even more compact, but rarely used short form of the periodic table .
Information content
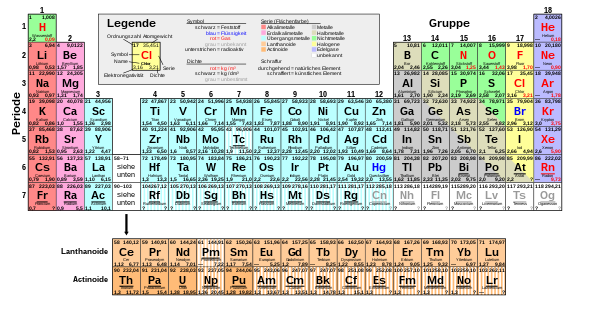
Usually the elements are listed with their atomic number and their element symbol . Depending on the application, further information on the element such as full name, mass, melting temperature, density and physical state can be given. Any information on “shells” relates to the shell model of atomic physics . Color codings are often used to represent different properties, for example the belonging to the metals, semi-metals or non-metals.
The peculiarity of the periodic table compared to a mere tabular listing of element properties, however, lies in the information about the relationships between the elements, which results from the placement of the elements concerned. The fact that an element belongs to a certain group immediately reveals the essential chemical characteristics of the element, such as its reactivity or preferred binding partners. The positioning within the overall system allows conclusions to be drawn regarding those properties that show a systematic trend in the periodic table, such as the ionization energy .
scope
With the most recent expansion of the periodic table in 2015, the elements 1 ( hydrogen ) to 118 ( oganesson ) have now been completely discovered or created and described. The elements of atomic numbers 1 to 94 occur in nature, whereby technetium (atomic number 43), promethium (61), astatine (85), neptunium (93) and plutonium (94) occur naturally in such small quantities that they are first artificial generated and described. Of these 94 natural elements, 83 are primordial , meaning that they have existed since the earth was formed. The original stocks of the other 11 have long since decayed because of their shorter half-lives, but they are constantly being rebuilt through radioactive decay in the natural decay series of the primordial elements.
The elements of ordinal numbers 95 to 118 were created exclusively artificially. The most recently discovered elements 113, 115, 117 and 118 were confirmed by the IUPAC on December 30, 2015 , which means that the seventh period of the periodic table is now complete.
Pictures of the respective elements can be found in the table of the chemical elements .
atomic structure
Structure of an atom
All substances are made up of atoms . An atom consists of protons and neutrons , which form the atomic nucleus , and of electrons , which surround the atomic nucleus as an " electron shell ". The protons each carry a positive and the electrons a negative elementary charge , so the number of electrons in the electron shell must be equal to the number of protons in the atomic nucleus if the atom is to be electrically neutral. The number of protons or electrons of an electrically neutral atom is called its " atomic number ".
Chemical compounds are substances made up of two or more types of atoms. The atoms combine to form molecules . The binding forces that hold the atoms together in a molecule are mediated by interactions between the electrons. The properties of the electrons in the outer area of the shell, the valence electrons, are decisive for the properties of the binding forces .
The chemical behavior of an atom - for example, its tendency to preferentially form compounds with certain other types of atoms - is thus largely determined by the structure of the electron shell and especially the valence electrons. This structure is always the same for a given number of electrons, so that the atomic number determines the chemical behavior of the atom.
Atoms with the same atomic number and therefore the same behavior in chemical reactions are called chemical elements . In the periodic table all existing elements are arranged in such a way that the laws resulting from the structure of the atoms in the chemical and atomic physical properties of the elements can be recognized.
Structure of the electron shell

The electron shell of an atom has structures that are examined and described by quantum mechanics . It can be divided into main shells. Each main shell can in turn be subdivided into subshells, which consist of orbitals . The quantum state where a given electron is by four quantum numbers described: The principal quantum number , the quantum number , the magnetic quantum number and the spin quantum number .
The main quantum number n = 1, 2, 3,… numbers the main shells. Alternatively, these shells can be referred to as K shell (for n = 1), L shell (for n = 2), M shell (for n = 3), and so on. The diameter of the main shells increases as the main quantum number increases.
A main shell with the main quantum number n has n sub-shells, which differ in their secondary quantum number. The lower shells are designated with the letters s , p , d , f and so on (the choice of these letters is due to historical reasons). A given subshell in a particular main shell is identified by its letter preceded by the main quantum number, for example 2p for the p subshell in the L shell ( n = 2).
The individual subshells are divided into orbitals, which are differentiated by the magnetic quantum number. Each s subshell contains one orbital, each p subshell contains three orbitals, each d subshell contains five orbitals, and each f subshell contains seven orbitals.
The spin quantum number describes the two possible spin orientations of the electron.
The Pauline Exclusion Principle states that no two electrons in an atom can match in all four quantum numbers. Two electrons that are in the same orbital already agree in three quantum numbers (namely those that describe this orbital). The two electrons must therefore differ in the fourth quantum number, their spin alignment. This exhausts the possible variations for the quantum numbers in this orbital, so each individual orbital can be occupied by a maximum of two electrons. The following maximum electron numbers result for the various shells:
- The K shell ( n = 1) has only one subshell ( 1s ) and this has only one single orbital. Since this can be occupied by a maximum of two electrons , the K shell accepts a maximum of two electrons.
- The L -shell ( n = 2) has two subshells 2s and 2p , which consist of one or three orbitals. It can hold a maximum of eight electrons in its four orbitals.
- The M -shell ( n = 3) has three subshells 3s , 3p and 3d , so it can hold a maximum of 18 electrons in its nine orbitals.
- The N shell ( n = 4) can hold a maximum of 32 electrons in its four subshells 4s to 4f and so on.
In general, a shell with the principal quantum number n can hold a maximum of 2 · n 2 electrons.
Systematic structure of the periodic table
In general, electrons that are on a higher main shell are more energetic than electrons on shells further inside. The main shells can, however, overlap energetically, since within a main shell the energy of the subshells increases in the sense of s → p → d → f and higher energy subshells of a given main shell can have a higher energy than the lowest energy subshells of the next main shell. This has consequences for the systematic structure of the periodic table.
The diagram opposite shows a schematic, not to scale representation of the energy levels in the electron shell. The lines on the left symbolize the main shells, the lines on the right their lower shells. The boxes represent the orbitals in each subshell, each of which can be assigned two electrons (“spin up” and “spin down”). From the main shell n = 3, the sub-shells of successive main shells overlap energetically.
If one imagines the atoms of the different elements to be generated one after the other in such a way that a proton in the nucleus and an electron in the shell (as well as the neutrons required, if applicable) are added to an atom of the previous element, then the added electron always occupies the lowest energy of the remaining free orbitals (" construction principle "). Since the occupation pattern of the individual orbitals repeats itself with the successive filling with the beginning of each new shell, the structures of the valence electrons repeat themselves and thus the chemical properties of the atoms.
For the sake of clarity, in the following text each element name is preceded by its ordinal number as an index. The color of the element boxes indicates the shell that is being refilled.
First period: 1 hydrogen to 2 helium
| 1 H. |
|
2 He |
| 1s lower shell | ||
The simplest atom is the 1 hydrogen atom, which has a proton in the nucleus and an electron in the shell (there are also isotopes with one or two neutrons). The electron is located in the s lower shell of the K shell.
This is followed by the 2 helium atom with two protons (as well as one or two neutrons) and two electrons. The added electron occupies the free space in the only orbital of the s lower shell. The K -shell is thus exhausted and the first period of the periodic table is filled.
Second period: 3 lithium to 10 neon
| 3 li |
4 Be |
|
5 B |
6 C |
7 N. |
8 O |
9 F. |
10 Ne |
| 2s - | 2p lower shell | |||||||
The L shell begins to be filled with the next electron : 3 Lithium has one electron in the 2s orbital, 4 Beryllium has a second electron in the 2s orbital, which is completely filled with it.
Now the filling of the 2 p orbitals begins : 5 Boron has an electron in the 2p orbital in addition to the filled 2s orbital. This is followed by 6 carbon, 7 nitrogen, 8 oxygen, 9 fluorine and 10 neon. The L -shell is completely filled with these eight elements and the second period ends.
Third period: 11 sodium to 18 argon
| 11 Well |
12 mg |
|
13 Al |
14 Si |
15 p |
16 pp |
17 cl |
18 ares |
| 3s - | 3p lower shell | |||||||
The filling of the M bowl begins with the same pattern. When looking at the respective configurations of the valence electrons, it is already clear that, for example, the first element of this period ( 11 sodium, with one valence electron) will have chemical similarities to the first element of the previous period ( 3 lithium, also with one valence electron).
Fourth period: 19 potassium to 36 krypton
| 19 K |
20 approx |
|
21 Sc |
22 Ti |
23 V |
24 Cr |
25 mn |
26 feet |
27 Co |
28 Ni |
29 Cu |
30 notes |
|
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 kr |
| 4s - | 3d lower shell | 4p subshell | |||||||||||||||||
After the eighth element of the third period, the 18 Argon, however, there will be an interruption in the regularity. By then, the 3s and 3p lower shells of the M shell were filled, and there are still ten spaces available in its 3d lower shell . However, since the 4s orbital of the next higher shell ( N , n = 4) has a lower energy than the 3d orbitals of the M shell, this 4s orbital is first filled with two electrons ( 19 potassium, 20 calcium). The 19 potassium has a valence electron and thus chemical similarity with 11 sodium and 3 lithium. Since the periodic table is supposed to highlight these and other similarities, a new period is started with the 19 potassium.
Only after 19 potassium and 20 calcium is the 3d lower shell of the M shell filled; this happens from 21 scandium to 30 zinc. These elements "inserted" in the periodic table all have a filled 4s lower shell and only differ in the degree of filling of the M shell below. They therefore show only relatively minor chemical differences; they belong to the "transition metals". With 30 zinc which is M -shell now completely full, is followed by the further filling of the remaining N -shell with the elements 31 Gallium up to 36 Krypton.
Fifth period: 37 rubidium to 54 xenon
| 37 Rb |
38 Sr |
|
39 Y |
40 Zr |
41 Nb |
42 Mon |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
|
49 in |
50 Sn |
51 Sb |
52 te |
53 I. |
54 Xe |
| 5s - | 4d lower shell | 5p lower shell | |||||||||||||||||
However, the filling of the N shell is interrupted again after the 36 krypton. The 4p lower shell is closed with the 36 krypton, and the lower shells 4d and 4f still need to be filled. Once again, however, the s -shell of the next higher shell ( O , n = 5 ) has a lower energy and is preferably filled up ( 37 rubidium, 38 strontium), which also allows a new period to begin again. This is followed by the ten transition metals 39 yttrium to 48 cadmium, with which the remaining 4d lower shell is filled, and then the six elements 49 indium to 54 xenon with which the 5p lower shell is filled.
Sixth period: 55 cesium to 86 radon
| 55 Cs |
56 Ba |
|
57 La |
|
72 Hf |
73 days |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 ed |
|
81 Tl |
82 Pb |
83 bi |
84 Po |
85 at |
86 para |
| 6s - | 5d - | 5d lower shell | 6p lower shell | |||||||||||||||||
| 58 Ce |
59 Pr |
60 Nd |
61 pm |
62 Sm |
63 Eu |
64 Gd |
65 p |
66 Dy |
67 Ho |
68 he |
69 Tm |
70 yb |
71 Lu |
|||||||
| 4f lower shell | ||||||||||||||||||||
This scheme, which is determined by the energetic position of the respective lower shells, is repeated in the following periods. In the sixth period the following sub-shells are filled one after the other: 6s ( 55 cesium and 56 barium), 5d ( 57 lanthanum), 4f ( 58 cerium to 71 lutetium), 5d ( 72 hafnium to 80 mercury) and 6p ( 81 thallium to 86 Radon).
In the diagram above, the filling of the 4f lower shell is shown as an inset to limit the width of the diagram.
Seventh period: 87 Francium to 118 Oganesson
| 87 Fr |
88 Ra |
|
89 Ac |
|
104 para |
105 Db |
106 Sg |
107 hours |
108 ms |
109 m |
110 Ds |
111 Rg |
112 cn |
|
113 Nh |
114 bottles |
115 Mc |
116 Lv |
117 Ts |
118 above |
| 7s - | 6d - | 6d lower shell | 7p lower shell | |||||||||||||||||
| 90 th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 am |
96 cm |
97 Bk |
98 Cf |
99 it |
100 m |
101 Md |
102 No. |
103 Lr |
|||||||
| 5f lower shell | ||||||||||||||||||||
In the seventh period the following are filled: 7s ( 87 Francium and 88 Radium), 6d ( 89 Actinium), 5f ( 90 Thorium to 103 Lawrencium), 6d ( 104 Rutherfordium to 112 Copernicium) and 7p ( 113 Nihonium to 118 Oganesson).
For the sake of simplicity, some irregularities when filling the individual sub-shells are not shown here. During the filling of the 4d shell, for example, one of the s electrons of some elements migrates into the d lower shell . For example, 47 silver does not have two electrons in the 5s lower shell and nine electrons in the 4d lower shell, as expected , but only one 5s electron and ten 4d electrons. A list of these exceptions can be found in the article on the structure principle .
In summary, the following filling pattern results (shown in the long form of the periodic table):
Filling the shell 1 H Hey K ( n = 1) 2 Li Be B. C. N O F. No L ( n = 2) 3 N / A Mg Al Si P S. Cl Ar M ( n = 3) 4th K Approx Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr N ( n = 4) 5 Rb Sr Y Zr Nb Mon Tc Ru Rh Pd Ag CD In Sn Sb Te I. Xe O ( n = 5) 6th Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho He Tm Yb Lu Hf Ta W. re Os Ir Pt Au Ed Tl Pb Bi Po At Marg P ( n = 6) 7th Fr. Ra Ac Th Pa U Np Pooh At the Cm Bk Cf It Fm Md No Lr Rf Db Sg Bra Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Above Q ( n = 7)
In representations of the periodic table, the periods are usually numbered consecutively with Arabic numerals from one to seven. The period number is also the main quantum number of the outermost main shell covered with electrons.
A main shell , while it is the outermost one, can only contain up to eight electrons (the K shell: up to two). The next electron added creates a new main shell, which now becomes the new outermost one. The main shell considered is only the second outermost, third outermost, and so on during its further filling. Regardless of the capacity of its outermost shell, each element only has between one and eight valence electrons.
Block structure of the periodic table
The systematic structure of the periodic table described above was done in such a way that the elements were arranged in the order of increasing ordinal numbers and a new line ("period") was started with certain elements. The criterion for the beginning of a new period was not the physical criterion of the degree of filling of the respective main shell, but the chemical similarity to the elements above it from the previous period, i.e. the same number of valence electrons. From this follows the structure of the periodic table, which is designed to make these connections visible. The following division of the periodic table into different blocks results :
Main groups
In the first two columns (“groups”) of the periodic table, the two orbitals of the s subshell of the current main shell are filled ( s block). In the last six groups, the six p subshells of the current main shell are filled ( p block). These eight groups are the main groups of the periodic table. From one main group to the next the number of valence electrons increases by one. For the 50 main group elements, the number of their valence electrons and thus their chemical behavior in its essential features is immediately apparent from their group membership. If the material properties of the elements are determined by the valence electrons, there are many similarities in the elements of the same group. The group number, usually written in Roman numerals, is also the number of electrons in the outermost main shell (with the exception of 2 helium, which is a noble gas in main group VIII, but has only two electrons).
The elements of the first main group each have a valence electron. With the exception of 1 hydrogen, they are soft, silvery-white and very reactive metals, the alkali metals . An example of the chemical similarity of the alkali metals is the fact that they all react with 17 chlorine to form colorless salts that crystallize in cube form. The formulas of these compounds also correspond to one another: LiCl, NaCl, KCl, RbCl, CsCl and FrCl.
The alkaline earth metals follow as the second main group. The boron group is the third main group, the carbon group the fourth and the nitrogen group the fifth. The chalcogens represent the sixth main group and the halogens the seventh.
As can be established quantum mechanically, not only closed main shells, but also closed lower shells are particularly stable. The elements in the eighth main group all have a closed main or sub-shell: In the case of 2 helium, the first main shell and thus also its only sub- shell 1s is completed. For the other elements 10 neon, 18 argon, 36 krypton, 54 xenon and 86 radon - if the main shell is not yet complete - the p -subshell is completed; these elements have eight valence electrons (one octet ). Because of the stability of their valence electron structures, these elements form almost no chemical bonds. They are all gaseous and are called noble gases .
Other elements can also achieve noble gas shells and thus particularly stable states by releasing or absorbing electrons. So the alkali metals easily give up their single valence electron and then appear as monovalent cations , for example 3 Li + , 11 Na + etc. The alkaline earth metals reach the noble gas configuration by releasing their two valence electrons and then form divalent cations, for example 4 Be ++ , 12 Mg ++ etc. The halogens, on the other hand, lack an electron to complete an octet. They therefore preferentially accept one electron, resulting in the monovalent anions 9 F - , 17 Cl - etc.
A block with subgroups is inserted between the main groups :
Subgroups: outer transition metals
In the last four periods, the filling of the respective outermost main shell was interrupted in order to fill the d -subshell of the second outermost main shell. The d subshells each hold 10 electrons, so there is an additional block with 10 groups in these four periods. All 40 elements in this d -block are metals, they are called "outer transition metals". They all have two valence electrons in the outermost shell (for exceptions see → construction principle ) and therefore show fewer differences in their chemical behavior than the main group elements. The existing differences are due to the different electronic structures of the next lower main shell. Corresponding to the repetitive filling pattern, the elements in this block also show clear similarities in their chemical properties.
Subgroups: inner transition metals
In the last two periods the filling of the d subshells of the second outermost main shell was also interrupted by the filling of the f subshells of the third outermost main shell. The f subshells each hold fourteen electrons, so there is an additional block with 14 groups in these two periods. The 28 elements in this f -block are called the inner transition elements . They have two valence electrons in the outermost main shell, one electron in the d -subshell of the penultimate main shell and only differ in the degree of filling of the third from last main shell (for exceptions see → construction principle ). Their chemical differences are correspondingly small.
The 14 inner transition metals from 58 cerium to 71 lutetium in the sixth period following 57 lanthanum are also called lanthanides . The 14 inner transition metals following 89 actinium from 90 thorium to 103 lawrencium in the seventh period are also called actinides .
Periodicities and Trends
Some properties of the elements vary in a systematic way with their position in the periodic table. If you move from one main group to the next within a period ("from left to right"), the physical and chemical properties change in a systematic, characteristic way, because the number of valence electrons increases by one at a time. In the next period, if the properties are determined by the number of valence electrons, they repeat in a similar manner because the number of valence electrons increases again in the same way.
If you move from one period to the next within a main group ("from top to bottom"), the properties in question are usually similar (same number of valence electrons), but gradually different (different main shells as the outermost shell).
Atomic radius
The atomic radius generally decreases within a period from left to right, because the electrons are drawn closer and closer to the nucleus due to the increasing atomic number . During the transition to the next period, the atomic radius increases sharply again because the occupation of the next outer main shell begins.
Within a group, the radius usually increases from top to bottom because a main shell is added in each case.
First ionization energy
The “first ionization energy ” is the energy that has to be expended to remove an electron from the electron shell, so that the neutral atom becomes a simply positively charged ion . The individual valence electron of the alkali metals is particularly loosely bound and can be easily detached. As the atomic number progresses within a period, an increasing amount of ionization energy must be expended until it reaches the maximum value of the period in the case of the noble gas with its particularly stable octet configuration.
Electron affinity
The electron affinity is the binding energy that is released when an atom binds an additional electron to itself, so that the neutral atom becomes a simply negatively charged ion. The halogens have a particularly high electron affinity because they can complete their electron octet by accepting an electron.
Electronegativity
If two atoms of different elements are chemically bound to each other, one of the two will usually attract the electrons of the common electron shell more strongly, so that the center of charge of the electron shell shifts towards this atom. The ability of an atom to attract electrons in a bond is measured by its electronegativity .
The electronegativity of the main group elements increases from left to right within a period because the nuclear charge increases. Within a group it usually grows from bottom to top, because in this direction the number of occupied main shells decreases and with it the shielding of the nuclear charge by the internal electrons. The element with the smallest electronegativity (0.7 according to Pauling) is 55 cesium , which is located at the bottom left in the periodic table . The element with the greatest electronegativity (4.0 according to Pauling) is 9 fluorine on the top right , followed by its left neighbor, 8 oxygen (3.5). 1 Hydrogen and the semi-metals occupy a middle position with values around 2. Most metals have values around 1.7 or less.
The shift in the center of charge in the molecule depends on the difference in the electronegativities of the two atoms. The more the center of gravity of the charge is shifted, the greater the ionic part of the bond, because the electrostatic attraction of the two unlike partial charges contributes to the bond all the more. The ionic bond character is particularly pronounced because of the described tendency towards electronegativities in bonds in which one bonding partner is on the left and the other on the right in the periodic table. An example of this is sodium chloride .
Bonds in which both partners come from the left half of the periodic table and therefore both belong to the metals ( see below ) are metallic bonds . Bonds where both partners come from the right side are mostly covalent bonds .
Value
One of the most characteristic features of an element is its value , i.e. its property to combine with certain preferred numbers of atoms of the various partner elements when a chemical compound is formed.
An atom that still lacks one electron to complete a valence electron octet can bond with a single 1 hydrogen atom in order to use the single valence electron of hydrogen in the shared electron shell to complete its own octet. An atom lacking two electrons will tend to connect with two 1 enter into hydrogen atoms. As these examples show, a connection between the preferred number of binding partners and the structure of the valence electron shell - that is, the group membership in the periodic table - is generally to be expected. However, the relationships are often much more complex than in the examples shown here.
A simple measure of the valency of an element is the number of 1 hydrogen atoms that the element binds to itself in a binary hydride . Another possible measure is twice the number of 8 oxygen atoms that the element binds in its oxide .
The elements of the first and the penultimate main group (the alkali metals or halogens) have the valence 1, so their hydrides have the formulas
- .
The elements of the second and third from last main group (the alkaline earth metals and the oxygen group) generally have the valence 2, so their hydrides are
- .
In the other main groups, the bonding possibilities are more diverse (there are countless hydrocarbon compounds), but one also encounters, for example, in the nitrogen group or and in the carbon group .
The 8 oxygen is divalent typical oxides of monovalent alkaline metals are therefore
and typical oxides of the divalent alkaline earth metals
- ,
but there are also other oxidation states . The last three oxides mentioned were the starting point for Döbereiner's triad system (see below).
Basicity
The basicity of the oxides and hydroxides of the elements increases from top to bottom and decreases from left to right. Oxides and hydroxides of metals dissolved in water (see below) form alkalis , while oxides and hydroxides of non-metals dissolved in water form acids .
Calcium oxide dissolved in water , for example, forms lime water . The same result is obtained by dissolving calcium hydroxide in water. Both sodium oxide and sodium hydroxide result in sodium hydroxide solution dissolved in water . Both potassium oxide and potassium hydroxide result in potassium hydroxide solution dissolved in water .
The metals from the first main group even dissolve as elements in water and result in basic ("alkaline") solutions. They are therefore called alkali metals. For example, sodium 11 dissolved in water produces sodium hydroxide solution, 19 potassium dissolved in water produces potassium hydroxide solution.
Carbon dioxide is an example of a non-metal oxide that, when dissolved in water, gives an acid, namely carbonic acid . Another example is sulfur trioxide , the aqueous solution of which is sulfuric acid .
Examples of further regularities
The most reactive elements are found in main groups I and VII (alkali metals or halogens), because these elements have a particularly strong tendency to release (with alkali metals) or take up (with halogens) an electron a complete octet of electrons to get.
The enthalpy of atomization, i.e. the energy that is required to break down a molecule E x formed from an element E , shows a clear periodicity for the main group elements depending on the also periodic cohesion of the elements, because the number x of bound atoms depends on this. The enthalpy of atomization shows minima for the 0-valent noble gases and maxima for the tetravalent elements of main group IV.
The density of the main group elements shows the same course because it is closely related to the bond of the respective element: The alkali metals have particularly small bonds and densities, the highest values are for the elements of the middle groups.
A similar pattern can be seen in the enthalpies of dissociation of E 2 molecules: The minima are again with the noble gases, the maxima now with the elements of main group V (N 2 , P 2 etc.), corresponding to the bonds possible with diatomic molecules.
The melting and boiling temperatures, the melting and evaporation heats are further examples of physical properties of the elements that show a periodic behavior. This even applies to the relevant properties of simple binary compounds, for example the melting temperatures or heats of fusion of the hydrides , fluorides , chlorides , bromides , iodides , oxides , sulfides and so on.
Metals, semi-metals and non-metals
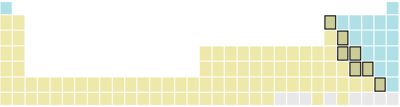
| H, C, N, P, O, S, Se, F, Cl, Br, I | He, Ne, Ar, Kr, Xe, Rn | ||
| H, C, N, P, O, S, (Se) | F, Cl, Br, I, At | He, Ne, Ar, Kr, Xe, Rn | |
| C, P, S, Se, I, At | Br | H, N, O, F, Cl, He, Ne, Ar, Kr, Xe, Rn | |
| H, C, P, S, Se, I | N, O, F, Cl, Br | He, Ne, Ar, Kr, Xe, Rn | |
| C, P, S, Se | H, N, O, F, Cl, Br, I | He, Ne, Ar, Kr, Xe, Rn | |
The vast majority of elements are metals . They are mostly silvery, shiny, malleable, not very volatile, and conduct electricity and heat. The metal character is most clearly pronounced in the elements in the lower left of the periodic table and decreases towards the upper right. The semimetals are connected in this direction (matt gray, glossy, brittle, slightly volatile, only moderately conductive and heat-conducting). At the top right of the periodic table are the non-metals (colored, not shiny, brittle, mostly volatile, not conductive and only poorly thermally conductive).
The first two main groups (the alkali and alkaline earth metals) therefore contain only metals, the last two main groups (the halogens and noble gases) only non-metals. The border between metals and non-metals marked by the semi-metals runs diagonally through the central main groups, so that these generally contain non-metals in the upper part, semi-metals below and metals in the lower part. Typical semimetals are 5 boron, 14 silicon or 32 germanium. Elements located on the border can even change their affiliation depending on the present modification : The 50 tin lying on the border between metals and semimetals is a metal as white β-tin , and a semimetal as gray α-tin . The lying on the boundary between the semi-metals and non-metals 6 carbon than graphite , a semi-metal, as a diamond , a non-metal.
In the V. and VI. Main group, the transition that takes place within a group can be clearly observed: The elements 7 nitrogen, 8 oxygen and 16 sulfur in the groups above are distinct non-metals. The subordinate elements 15 phosphorus, 33 arsenic and 34 selenium occur both in non-metallic modifications ( white , red and purple phosphorus , yellow arsenic , red selenium ) as well as in semiconducting modifications ( black phosphorus , gray arsenic , gray selenium ). The elements 51 antimony, 52 tellurium, 83 bismuth and 84 polonium in the groups below occur preferentially in semi-metallic or metallic form.
The typical representatives of the metals on the left-hand side of the periodic table always only have a small number of valence electrons and willingly give them up (low ionization energy, see above ) in order to achieve an octet of valence electrons. When metal atoms combine to form a metal lattice by means of chemical bonds, the released valence electrons form an "electron gas" that embeds the positively charged metal atoms and holds them together. This is what is known as the metallic bond . From the properties of this type of bond, the characteristic properties of the metals, such as their shine or their easy ductility, follow. In particular, the large number of freely moving electrons leads to high electrical conductivity.
More complex relationships
Special position of the head elements
The periodic table arranges the elements in such a way that the elements belonging to a group are chemically and physically similar to one another. The degree of similarity varies from case to case, but it is noticeable that the first members of each main group (the "head elements" Li, Be, B, C, N, O, F) have less similarity with the rest of the members of their group have than these respectively among themselves. Reasons for this are, among other things, that due to the small atomic radii, the valence electrons of these atoms are particularly strongly bound to the nuclei, and that the head elements, unlike the other group members in the outer shell, cannot exceed an electron octet.
An example of this special position is the gaseous form of 7 nitrogen and 8 oxygen in contrast to other representatives of the respective group. Another example is the fact that the head elements cannot assume any higher oxidation numbers than their valence electron structure corresponds to. Thus, the 8 oxygen can assume at most the oxidation number +2, while the other members of the oxygen group often have the oxidation numbers +4 and +6, which they achieve through the participation of the oxygen-missing d orbitals in the respective bond.
The special position of the head element is particularly pronounced in the s block of the periodic table (especially if one counts the 1 hydrogen instead of the 3 lithium as the head element), less pronounced in the p block, although it is present but only slightly pronounced in the d block and even less in the f block.
Oblique relationships
The named head elements resemble the main group elements on the right below them in the periodic table more than their own group members and are therefore examples of oblique relationships . This applies in particular to similarities between 3 lithium and 12 magnesium, 4 beryllium and 13 aluminum, 5 boron and 14 silicon. The reason for this is that some important trends in elementary properties such as electronegativity, ionization energy or atomic radii run from bottom left to top right and thus “obliquely” in the periodic table. For example, if you move down the periodic table, the electronegativity decreases. If you move to the right, it increases. When moving down to the right, the two trends approximately cancel each other out and the electronegativity is only slightly changed.
Another oblique relationship is Grimm's hydride shift theorem .
Knight relationship
An unusual relationship between elements is the knight relationship according to Michael Laing , which is characterized in analogy to the chess piece of the knight in that some metallic elements from the fourth period in some characteristics (e.g. melting points and boiling points ) have similar properties to a metallic one Have element one period below and two groups to the right. Examples are 30 zinc and 50 tin, which have the same properties in an alloy with copper, in the coating of steel and in their biological importance as a trace element . Further examples are 48 cadmium and 82 lead, 47 silver and 81 thallium, and 31 gallium and 51 antimony.
Relationships between main and subgroups
There are many similarities between a given group n and group n + 10 ten columns to the right . A striking example are 12 magnesium from the second and 30 zinc from the twelfth group, whose sulfates, hydroxides, carbonates and chlorides behave very similarly. Other distinct examples are 21 scandium from the third group and 13 aluminum from the thirteenth group, as well as 22 titanium from the fourth group and 50 tin from the fourteenth group. Only between the alkali metals in the first group and the precious metals ( 29 copper, 47 silver, 79 gold) in the eleventh group is there no similarity.
In the medium-length form of the periodic table in use today, these relationships are not very obvious. However, they were well known to the early pioneers of the periodic table, who could only orientate themselves on chemical similarities. The relationships mean that the "long" periods four to seven (without the lanthanoids and actinides shown separately) have a double periodicity: both their left half (up to the precious metals) and their right half (up to the noble gases) Properties that tend to run in parallel with the main groups in the short periods two and three.
The so-called short period system takes these similarities into account by presenting the two short periods two and three as a closed block (not divided into two as is otherwise the case), while it divides the four long periods and lists their left and right halves as separate lines below one another. For this purpose, the elements of the iron, cobalt and nickel groups are combined into one group in each of the long periods containing 18 elements . These periods can then be divided into two halves of eight groups each (one of which is a group of three), which are arranged one below the other in the short period system. The short period system therefore only has 8 columns. Due to the existence of a group of three, however, despite the eight columns, the transition to the element one row lower corresponds to the transition to the element ten groups further to the right in the long form . The original main and subgroups can be distinguished by indenting them differently.
The short form of the periodic table shows in particular the parallel course of the valencies (more precisely: the maximum oxidation numbers) between secondary and main groups , which has been lost in the long forms and only survives there in the form of group numbering (see next section). On the other hand, the short form is less clear than the long forms, and it also emphasizes the similarities between main and subgroups more than they actually are.
Excursus: numbering the groups
| 1 | 2 | 3 | 4th | 5 | 6th | 7th | 8th | 9 | 10 | 11 | 12 | 13 | 14th | 15th | 16 | 17th | 18th |
| Yes | IIa | IIIb | IVb | Vb | VIb | VIIb | VIIIb | Ib | IIb | IIIa | IVa | Va | Via | VIIa | VIIIa | ||
| Yes | IIa | IIIa | IVa | Va | Via | VIIa | VIIIa | Ib | IIb | IIIb | IVb | Vb | VIb | VIIb | VIIIb | ||
| H | Hey | ||||||||||||||||
| Li | Be | B. | C. | N | O | F. | No | ||||||||||
| N / A | Mg | Al | Si | P | S. | Cl | Ar | ||||||||||
| K | Approx | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mon | Tc | Ru | Rh | Pd | Ag | CD | In | Sn | Sb | Te | I. | Xe |
| Cs | Ba | * | Hf | Ta | W. | re | Os | Ir | Pt | Au | Ed | Tl | Pb | Bi | Po | At | Marg |
| Fr. | Ra | * | Rf | Db | Sg | Bra | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Above |
Two of the three common numbering systems for groups go back to the group arrangement in the short period system just described.
The eight groups of the short period system are numbered from I to VIII using Roman numerals. If one pulls the short period system apart again into the long form, the main and sub-group elements combined in a group of the short form must be divided again into two separate groups in the long form. If you want to keep the group numbering in the short form, each group number is doubled. To differentiate, add an a or b to the group number.
In the convention, which is mainly used in the USA, the main group elements receive an a, the subgroup elements a b. The result is the numbering sequence (main groups shown in bold):
- Ia IIa IIIb IVb Vb VIb VIb VIIIb Ib IIb IIIa IVa Va VIa VIIa VIIIa
In the convention mainly used in Europe, the first series from I to VIII is consistently given an a, the second a b. The result is the numbering sequence
- Ia IIa IIIa IVa Va VIa VIIa VIIIa Ib IIb IIIb IVb Vb VIb VIIb VIIIb
The advantage of the two numbering systems derived from the short form is that the group number for the main groups is identical to the number of valence electrons. It is therefore immediately apparent that, for example, the elements of main group IV have four valence electrons.
The IUPAC recommends numbering the groups sequentially with Arabic numerals from 1 to 18. While this numbering is transparent and unambiguous, the relationship between the group number and the number of valence electrons is lost. For example, the elements with four valence electrons are in group 14.
Additional influences
The properties of unknown elements can be approximately predicted if the properties of the surrounding elements in the periodic table are known. The regular variation of the properties within the groups and periods is, however, interrupted by numerous exceptions, which add to the complexity of the field of chemistry. The higher the atomic number, the less suitable the systematics of the periodic table is for predicting material properties, since the higher charge of the atomic nucleus increases the speed of electrons close to the nucleus and thus the relativistic effects . In elements from the fourth period onwards, the electrons of the innermost shells (especially the s orbitals) move closer to the atomic nucleus due to the increasing number of positive charges in the atomic nucleus, which means that the speed of these electrons almost reaches the speed of light . As a result, contrary to the general tendency, the ion radius decreases and the ionization energy for these electrons increases ( effect of the inert electron pair ).
Radioactive elements
The radioactive elements are marked as additional information that has nothing to do with the electron configuration and therefore with the position in the PSE:
Element 82 (lead) is the last element of which stable, i.e. non-radioactive, isotopes exist. All of the following (atomic number 83 and higher) have radioactive and thus unstable isotopes without exception . 83 (bismuth) is a borderline case. It only has unstable isotopes, including one with an extremely long half-life. Also within elements 1 to 82 there are two substances that have only unstable isotopes: 43 (technetium) and 61 (promethium).
This actually leaves only 80 stable elements that occur in nature - all the others are radioactive elements. Of the radioactive elements, only bismuth, thorium and uranium are found in nature in large quantities, since these elements have half-lives in the order of magnitude of the age of the earth or longer. With the exception of one isotope of plutonium, all other radioactive elements are either, like radium, intermediate decay products of one of the three natural radioactive decay series or arise from rare natural nuclear reactions or from spontaneous splitting of uranium and thorium. Elements with ordinal numbers above 94 can only be produced artificially; Although they are also formed during element synthesis in a supernova , no traces of them have been found in nature due to their short half-lives. The last element detected so far is Oganesson with the ordinal number 118, but this only has a half-life of 0.89 ms. Presumably there is an island of stability with higher ordinal numbers .
Atomic masses
Since the number of protons in the atomic nucleus is identical to the atomic number of the atom, the atomic mass increases with the atomic number. While the atomic number increases by one unit from one element to the next, the increase in the atomic mass is much more irregular.
The mass of a proton is 1.0073 atomic mass units (1 u = 1.66 · 10 −27 kg), that of a neutron is 1.0087 u. The mass of an electron of 0.0005 u is mostly negligible. The mass of a hydrogen atom consisting of a proton and an electron is 1.0078 u. Since all atoms have an integer number of protons and neutrons (each with about 1 u mass) in the nucleus, they basically also have an integer atomic mass to a good approximation, which corresponds to the number of protons and neutrons contained in the nucleus (which are atomic masses usually a little smaller than a whole number, the " mass defect " corresponds to the binding energy released during the formation of the core). In the apparent contradiction to this, however, some of the mass data in the periodic table differ significantly from the integer. For the chlorine, for example, there is the indication 35.45 u.
| ele- ment |
Mass number (isotope) |
Natural frequency |
Atomic mass (u) |
Mean atomic mass (u) |
|---|---|---|---|---|
| ... | ... | ... | ... | ... |
| 15 p | 31 | 100% | 30.97 | 30.97 |
| 16 pp | 32 | 95.02% | 31.97 | 32.06 |
| 33 | 0.75% | 32.97 | ||
| 34 | 4.21% | 33.97 | ||
| 36 | 0.02% | 35.97 | ||
| 17 cl | 35 | 75.77% | 34.97 | 35.45 |
| 37 | 24.23% | 36.97 | ||
| 18 ares | 36 | 0.337% | 35.97 | 39.95 |
| 38 | 0.063% | 37.96 | ||
| 40 | 99.600% | 39.96 | ||
| ... | ... | ... | ... | ... |
The reason is that two atoms with the same number of protons can have different numbers of neutrons. Such atoms have the same atomic number and thus the same chemical behavior, so by definition belong to the same chemical element and are therefore in the same place in the periodic table. But because they have different numbers of neutrons, they are different “ isotopes ” of this element (from ancient Greek ἴσος ísos “equal” and τόπος tópos “place, place”).
The 20 elements
consist of only one naturally occurring isotope, they are pure elements . The other elements are mixed elements ; in their natural occurrence they consist of a mixture of different isotopes. For these mixed elements, the mean atomic mass of the naturally occurring isotope mixture is entered in the periodic table . The naturally occurring chlorine, for example, consists of 75.77% of the chlorine isotope with the mass number 35 (with 17 protons and 18 neutrons in the nucleus) and 24.23% of the chlorine isotope 37 (17 protons and 20 neutrons). Its mean atomic mass is the frequency-weighted mean of the (almost integer) atomic masses 34.97 u and 36.97 u, i.e. the above-mentioned 35.45 u.
If the isotopes of two successive elements in the periodic table have very different frequency distributions, it can happen that the mean atomic mass decreases from one element to the next. Thus, the 19 potassium following the 18 argon has a higher atomic number, but a lower average atomic mass. The same applies to 27 cobalt and 28 nickel, 52 tellurium and 53 iodine, and 90 thorium and 91 protactinium.
Since the atomic mass (apart from the mentioned exceptions) grow more or less regularly with the ordinal number, in the 19th century they could successfully be used instead of the actual principle of order, the as yet unknown ordinal number, the search for laws.
history

elements
In ancient Greece and in ancient China it was speculated more than 2000 years ago that the multitude of phenomena in nature must be traced back to a small number of "elements". In Greece Empedocles represented the four-element doctrine with the elements fire , water , earth and air . In China there were the elements wood , fire, earth, metal and water in the five-element teaching .
The current concept of an element as a substance that cannot be further broken down goes back to Joachim Jungius and Robert Boyle . In 1789, Antoine Laurent de Lavoisier presented the first systematic table comprising 33 entries with presumed “simple substances”, of which 21 were in fact already elements in today's sense. However, there was still complete uncertainty about the internal structure of the elements and thus all matter in general. According to John Dalton's atomic hypothesis (1808), all substances are made up of the smallest, indivisible “atoms”, whereby the atoms of one chemical element are identical to one another, but differ in shape and weight from the atoms of another element. According to the hypothesis, chemical reactions were to be regarded as regrouping of indestructible atoms, and the laws of constant proportions and multiple proportions could also be easily explained. Although atoms were accepted as a working hypothesis by many chemists, there was no evidence of their existence.
Atomic masses
While the densities of the various elements had been known for a long time, the lack of knowledge about the number and size of the atoms made it impossible to determine their absolute masses. Dalton had already drawn up a 14-element and still rather imprecise list of the ratios of the atomic masses based on constant proportions.
William Prout noticed that many atomic masses were roughly integer multiples of the atomic mass of hydrogen and in 1815 he hypothesized that all elements were composed of corresponding amounts of hydrogen as the "original substance". The atomic masses previously listed as non-whole numbers would turn out to be whole numbers on more precise measurements. Prout's hypothesis caused more precise mass determinations, mainly by Jöns Jakob Berzelius and Jean Servais Stas , who confirmed the non-integer nature of many atomic masses and thus refuted Prout's hypothesis, but also served as the basis for more reliable investigations because of their significantly improved accuracy. The reason for the strikingly large number of elements with approximately whole-numbered atomic masses remained unclear.
In the 1850s attacked Stanislao Cannizzaro by Amedeo Avogadro drawn up in 1811, but so far remained unnoticed hypothesis again that equal volumes of different gases contain at the same temperature and pressure, the same number of particles. This hypothesis made it possible to systematically compare the masses of equal (albeit unknown) numbers of atoms in gaseous compounds and to determine the relative atomic masses of the elements with reference to a reference element. With their help, numerous previously incorrectly assumed proportions in chemical compounds could be corrected. On this basis, Cannizzaro published more reliable and consistent atomic masses between 1858 and 1860 in preparation for the Karlsruhe Congress (which Meyer and Mendeleev also attended), which enabled rapid progress in the development of periodic systems in the 1860s.
Precursor to the periodic table
At the beginning of the 19th century, regularities in the relationships between the elements were sought. Obstacles were, among other things, the uncertainties in the atomic mass and the fact that numerous elements were not even known. For the first time, Döbereiner established a connection between atomic mass and the chemical properties of individual elements. In 1824 Falckner published a system of natural element families. In 1843 Gmelin created a tabular sorting of the elements. Other pioneers, who also knew Mendeleev, were Pettenkofer (1850), Odling (1857), Dumas (1858) and Lenßen (1857). In 1862, Chancourtois developed a three-dimensional representation, in which he arranged the elements helically on a cylinder according to increasing atomic masses. Attempts were also made by Hinrichs (1864), Baumhauer (1867) and Quaglio (1871) to depict the system in a spiral. In 1863/64 Newlands put up a table of the elements in groups of eight (law of octaves) arranged according to atomic mass.
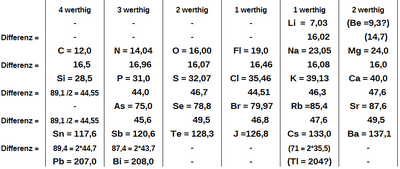
Johann Wolfgang Döbereiner (Triad System)
Johann Wolfgang Döbereiner made the first attempt to organize elements according to recognized laws. In 1817 he determined the molecular mass of strontium oxide and found (in the mass system he used) the value 50. Döbereiner noticed that this was exactly the arithmetic mean of the masses of calcium oxide (27.5) and barium oxide (72.5) :
From this he initially drew the suspicion that strontium consisted of barium and calcium, which he did not find confirmed in corresponding experiments.
From a modern point of view, calcium, strontium and barium are three elements from the group of alkaline earth metals that are one below the other in the periodic table, which explains their identical valencies and therefore their similarity in the formation of oxides . Since the period length in this area of the periodic table is 18 elements (a period here comprises eight main groups and ten subgroups), they show the same difference in ordinal numbers among each other (18, Döbereiner still unknown):
and therefore roughly the same difference in atomic masses (just under 50 u).
In 1827, Leopold Gmelin, in his “Handbuch der Theoretischen Chemie” (Handbook of Theoretical Chemistry), noted “some strange relationships with atomic masses, which are undoubtedly related to the innermost nature of substances.” Among other things, he pointed to another group of three, namely lithium, sodium and potassium. If one takes the arithmetic mean of the atomic masses of lithium and potassium, "one obtains fairly precisely [the atomic mass] of the sodium, which metal comes into its chemical relationship between the two mentioned."
| H | Hey | ||||||||||||||||
| Li | Be | B. | C. | N | O | F. | No | ||||||||||
| N / A | Mg | Al | Si | P | S. | Cl | Ar | ||||||||||
| K | Approx | ... | Ga | Ge | As | Se | Br | Kr | |||||||||
| Rb | Sr | ... | In | Sn | Sb | Te | I. | Xe | |||||||||
| Cs | Ba | ... | Tl | Pb | Bi | Po | At | Marg | |||||||||
| Fr. | Ra | ... | Nh | Fl | Mc | Lv | Ts | Above | |||||||||
| Position of the four triads in the modern periodic table | |||||||||||||||||
In 1829 Döbereiner published a more detailed “attempt to group elementary substances according to their analogy”. A newly discovered triad contained "three salt formers" with chlorine and iodine as well as bromine, which was only isolated the previous year. The comparison using the atomic masses determined by Berzelius gave
Another newly found triad comprised sulfur, selenium and tellurium, all of which "combine with hydrogen to form peculiar hydrogen acids":
In his attempts at order, Döbereiner insisted that the elements combined into a triad actually showed chemical similarity: “The fact that the arithmetic mean of the atomic weights of oxygen = 16.026 and carbon = 12.256 expresses the atomic weight of nitrogen = 14.138 can be used here cannot be considered because there is no analogy between these three substances. ”He also insisted on the special meaning of the number three. The elements iron, manganese, nickel, cobalt, zinc and copper, which are very similar to one another, presented a problem for him, because "how should they be arranged if the triad is accepted as the principle of grouping?"
| O | N | H | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F. | Cl | Br | J | L. | N / A | K | |||||||||||
| S. | Se | Te | Mg | Approx | Sr | Ba | |||||||||||
| P | As | Sb | G | Y | Ce | La | |||||||||||
| C. | B. | Si | Zr | Th | Al | ||||||||||||
| Ti | Ta | W. | Sn | CD | Zn | ||||||||||||
| Mon | V | Cr | U | Mn | Co | Ni | Fe | ||||||||||
| Bi Pb Ag Hg Cu | |||||||||||||||||
| Os Ir R Pt Pd Au | |||||||||||||||||
In 1827 Gmelin had shown the then known 51 elements individually in a V-shaped arrangement in order to clearly show their "relationship and difference". In 1843 he went over to grouping the 55 known elements "according to their physical and chemical conditions" mostly to summarize three elements, which in turn were arranged "according to their similarities" in a V-shaped scheme in order of increasing electropositivity. Today's main groups can be recognized in some of Gmelin's groups ( R = rhodium, today Rh; L = lithium, today Li; G = glycium, today beryllium Be).
In 1857 Ernst Lenßen was able to divide practically all elements known at the time into 20 triads (but was less strict than Döbereiner with regard to chemical similarity). He even put groups of three of triads together to form "enneads" (groups of nine), in which the atomic masses of the respective middle triads were in turn related by the mean value rule. Using his system, he predicted, among other things, the atomic masses of the elements erbium and terbium, which had already been discovered but not yet isolated, but none of his predictions were successful. He also tried to make connections with other physical and chemical properties.
John AR Newlands (Law of Octaves)
The attempts at order to date have been largely limited to finding isolated groups with similar elements. In 1864 John Alexander Reina Newlands published a table with 24 elements (and a space for a supposedly still undiscovered element) in which the elements were arranged as usual in the order of increasing atomic masses, but in which he did not refer to patterns in the atomic mass differences , but pointed to repeated differences in the place numbers of similar elements. This was the first periodic system , that is, a compilation of elements that shows that the properties of the elements repeat themselves after certain regular intervals. Newlands was also the first to swap the order of the elements iodine and tellurium based on atomic mass and to prefer the order based on chemical properties.
In 1865, Newlands developed another system that now comprised 65 elements. It should show that the chemical properties are repeated in every eighth position, which he compared to the octaves in music. (Since the noble gases had not yet been discovered, the period length in the first periods of Newland's table was actually seven elements. But since he counted both similar elements, just like the octave in music, for example, from a C to counts the next C inclusive, results in a period length of 8.)
Newlands called this relationship between the elements the "law of octaves", which was the first time that the repetition pattern in the element properties was considered a law of nature. The law of octaves can be applied perfectly to the first two periods, but because then (as we know today) the periods become longer, the law was less successful in the following periods.
The first accurate prediction of an as yet undiscovered element goes back to Newlands: Due to a gap in one of his tables, he predicted the existence of an element with atomic mass 73 between silicon and tin in 1864. In the announced position and with an atomic mass of 72.61, this corresponds to the germanium discovered in 1886. However, his predictions of still unknown elements between rhodium and iridium and between palladium and platinum did not come true.
The discovery of periodicity is occasionally attributed to Alexandre-Emile Béguyer de Chancourtois, who in 1862 arranged the elements according to increasing atomic mass along a three-dimensional screw in such a way that a screw turn corresponded to 16 units, i.e. elements at a distance of 16 units came to stand vertically one above the other. However, his system went largely unnoticed and he did not develop it further.
Dmitri Mendeleev and Lothar Meyer (periodic table)
The modern periodic table was developed by Lothar Meyer and Dmitri Iwanowitsch Mendelejew . Both published their results in 1869 and in 1882 they jointly received the Davy Medal of the British Royal Society for their work .
Mendeleev is mentioned more often than Meyer as the founder of today's periodic table. On the one hand, because Meyer's periodic table was published a few months later, on the other hand, because Mendeleev made predictions about the properties of the as yet undiscovered elements. In Russia the periodic table is still called Tabliza Mendeleeva ("Mendeleev's table"). Neither Mendeleev nor Meyer knew each other's work on the periodic table. The works of Béguyer de Chancourtois from 1862, Newlands from 1863/64 or Hinrichs from 1866/67 were also unknown to Mendeleev.
Lothar Meyer
In his textbook "Die modern Theorien der Chemie" , published in 1864, Meyer already presented a table containing 28 elements and arranged according to increasing atomic masses. The division into rows was made in such a way that each column (corresponding to today's main groups) contained elements of the same value and the value changed by one unit from one column to the next. Meyer pointed out that the atomic mass difference between the first and second elements of each column was about 16, the next two differences fluctuated around 46, and the last difference was always about 87 to 90. He suggested that - similar to homologous series of molecules - this could indicate the systematic structure of the atoms from smaller components.
Meyer had exchanged the elements tellurium and iodine, according to their chemical properties, in relation to the sequence based on the atomic masses. Meyer had to leave some gaps in the table, including one between silicon and tin, in which, according to his difference scheme, an element of atomic mass 73 was to be expected. The missing element was germanium, discovered in 1886, with an atomic mass of 72.61. Another table, not sorted according to atomic mass, contained 22 elements that Meyer had not included in his scheme - they correspond to today's transition metals.
In 1870 (submitted in December 1869, barely a year after Mendeleev's first publication of a periodic table) Meyer published an expanded version of his table in which he had succeeded in using updated atomic masses to “classify all elements that were sufficiently well known in the same scheme”. The periods in this system ran vertically, the groups horizontally. The (not yet so called) transition metals were now part of the table. Similar to a short period system, they were arranged in periods that alternated with the (not yet so-called) main groups.
To illustrate the variation of the properties along the periods, Meyer added a diagram that shows the periodically varying atomic volumes as a function of the atomic mass (similar to the diagram in the section Atomic Radii ). This illustration contributed significantly to the acceptance of the periodic table. Meyer discussed various physical properties of the atoms that run parallel to the atomic volumes and are therefore also periodic, such as densities, volatility, ductility, brittleness or specific heat.
Dmitri Mendeleev
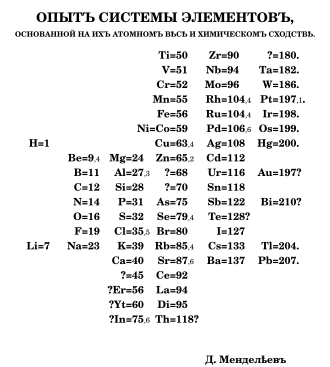
Mendeleev's name is mainly associated with the periodic table in its present form. His periodic table was more complete than other systems of that time, he actively promoted and defended his system, refined it over decades and used it for far more extensive and detailed predictions than other periodic system authors.
In search of a structure for his chemistry textbook, Mendeleev created Jul. / March 1, 1869 greg. a first draft of his version of the periodic table. In March he published his system with a detailed explanation in the journal of the Russian Chemical Society.
He expressly pointed out that most of the properties of the elements are not suitable as a clear ordering principle. For example, most elements can have different values. Most of the properties of the free elements depend on the modification present (graphite and diamond, for example, are modifications of carbon with significantly different properties), and so on. The only unambiguous and numerically measurable property of an element, which is preserved in all modifications of the free element as well as in all its compounds, is its atomic mass (the atomic number as another such property was still unknown to Mendeleev).
He arranged the elements of the "natural groups" already known as belonging together (such as the halogens, the alkaline earth metals, the nitrogen group, etc.) according to their atomic mass and found that this arrangement corresponded to "the natural resemblance prevailing among the elements" without further ado. He stated: "The elements arranged according to the size of their atomic weight show a clear periodicity of their properties," and on this basis tried to fit the other elements into the scheme according to their chemical behavior.
In this article, Mendeleev already predicted the existence of two new elements with atomic masses between 65 and 75, which were supposed to resemble aluminum and silicon, based on gaps that had remained in his system. Like some of his predecessors, Mendeleev had swapped tellurium and iodine in relation to the sequence derived from the atomic masses. His prediction that the atomic mass of tellurium had to be corrected because according to his system it could not be 128 and must rather be between 123 and 126 did not come true - here there is actually an irregularity of the atomic masses. In the same year, two short German-language descriptions of the new system were published.
In 1871 an extensive article appeared in which Mendeleev presented two further developed variants of his periodic table. One of these variants was the first short period system. In this article he demonstrated, among other things, how the atomic mass of an element could be determined or corrected using the periodic table if its chemical behavior was known. The article also contains the three most famous predictions about the properties of as yet unknown elements, the existence of which Mendeleev discovered from remaining gaps in his periodic table. By clever interpolation between the physical and chemical properties of the neighboring elements, he was able to accurately predict numerous properties of the as yet unknown elements.
Mendeleev named the unknown elements after the element above the respective gap in his short period system with the addition of the prefix Eka ( Sanskrit: "one"). Ekaaluminium was discovered by Paul Émile Lecoq de Boisbaudran in 1875 and named gallium after France, the land of discovery . Ekabor was discovered in 1879 by Lars Fredrik Nilson and - after Scandinavia - given the name Scandium. Ecasilicon was discovered by Clemens Winkler in 1886 and was named germanium after the country of discovery, Germany .
| element | oxide | chloride | Ethyl compound | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atomic mass |
Density (g / cm³) |
Heat cap. J / (kg K) |
colour | formula | Density (g / cm³) |
formula | Boiling point |
Density (g / cm³) |
formula | Boiling point |
|
| forecast | 72 | 5.5 | 306 | dark gray | EsO 2 | 4.7 | EsCl 4 | 100 ° C | 1.9 | Es (C 2 H 5 ) 4 | 160 ° C |
| found | 72.32 | 5.47 | 318 | grayish-white | GeO 2 | 4.703 | GeCl 4 | 86 ° C | 1,887 | Ge (C 2 H 5 ) 4 | 160 ° C |
Not all of Mendeleev's predictions were that successful. Overall, only about half of his predictions of new elements were true.
The noble gas argon, discovered in 1894, appeared to pose a significant threat to the general validity of Mendeleev's periodic table, as it could not be integrated into the existing system. However, when other noble gases were discovered in rapid succession (1895 helium, 1898 neon, krypton and xenon, 1900 radon) it became apparent that the periodic table only had to be expanded to include a new group of elements between halogens and alkali metals in order to be able to include them all . Mendeleev spoke of a “critical test” that his periodic table “survived brilliantly”.
Mendeleev published about thirty versions of the periodic table over the years, and another thirty are available in manuscript. The oldest surviving display board in the periodic table dates from 1879 to 1886 and is located in the University of St. Andrews .
| Periodic table according to Mendeleev, 1869 | Modern periodic table to uranium, arranged according to Mendeleev's scheme | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc = 45 | Y = 89 | La - Lu | Ac, Th, Pa, U | |||||||||||
| Ti = 50 | Zr = 90 | ? = 180 | Ti = 48 | Zr = 91 | Hf = 178 | |||||||||
| V = 51 | Nb = 94 | Ta = 182 | V = 51 | Nb = 93 | Ta = 181 | |||||||||
| Cr = 52 | Mo = 96 | W = 186 | Cr = 52 | Mo = 96 | W = 184 | |||||||||
| Mn = 55 | Rh = 104.4 | Pt = 197.4 | Mn = 55 | Tc = 97 | Re = 186 | |||||||||
| Fe = 56 | Ru = 104.4 | Ir = 198 | Fe = 56 | Ru = 101 | Os = 190 | |||||||||
| Ni = 59 Co = 59 |
Pd = 106.6 | Os = 199 | Co = 59 | Rh = 103 | Ir = 192 | |||||||||
| Ni = 59 | Pd = 106 | Pt = 195 | ||||||||||||
| H = 1 | Cu = 63.4 | Ag = 108 | Hg = 200 | Cu = 64 | Ag = 108 | Au = 197 | ||||||||
| Be = 9.4 | Mg = 24 | Zn = 65.2 | Cd = 112 | Zn = 65 | Cd = 112 | Hg = 201 | ||||||||
| B = 11 | Al = 27.4 | ? = 68 | Ur = 116 | Au = 197? | B = 11 | Al = 27 | Ga = 70 | In = 115 | Tl = 204 | |||||
| C = 12 | Si = 28 | ? = 70 | Sn = 118 | C = 12 | Si = 28 | Ge = 73 | Sn = 119 | Pb = 207 | ||||||
| N = 14 | P = 31 | As = 75 | Sb = 122 | Bi = 210? | N = 14 | P = 31 | As = 75 | Sb = 122 | Bi = 209 | |||||
| O = 16 | S = 32 | Se = 79.4 | Te = 128? | O = 16 | S = 32 | Se = 79 | Te = 128 | Po = 209 | ||||||
| F = 19 | Cl = 35.5 | Br = 80 | J = 127 | F = 19 | Cl = 35 | Br = 80 | I = 127 | At = 210 | ||||||
| Li = 7 | Na = 23 | K = 39 | Rb = 85.4 | Cs = 133 | Tl = 204 | He = 4 | Ne = 20 | Ar = 40 | Kr = 84 | Xe = 131 | Rn = 222 | |||
| Ca = 40 | Sr = 87.6 | Ba = 137 | Pb = 207 | H = 1 | Li = 7 | Na = 23 | K = 39 | Rb = 85 | Cs = 133 | Fr = 223 | ||||
| ? = 45 | Ce = 92 | Be = 9 | Mg = 24 | Ca = 40 | Sr = 88 | Ba = 137 | Ra = 226 | |||||||
| ? He = 56 | La = 94 | |||||||||||||
| ? Yt = 60 | Di = 95 | |||||||||||||
| ? In = 75.6 | Th = 118? | |||||||||||||
The colors indicate the current assignment of the elements:
Alkali metals, Alkaline earth metals, 3rd main group, 4th main group, 5th main group,6th main group, Halogens, Noble gases, Transition metals, Lanthanoids, Actinoids. The supposed element didymium (Di) later turned out to be a mixture of the rare earths praseodymium and neodymium. In order to convert the modern periodic table shown on the right from Mendeleev's arrangement into the order common today, the last two lines are to be added at the top, shifted one box to the right, and the whole system is mirrored on the diagonal running from top left to bottom right. In the modern periodic table shown, the atomic masses are rounded to whole numbers for the sake of clarity.
Henri Becquerel (radioactivity)
Henri Becquerel discovered in 1896 that uranium emitted a previously unknown radiation. The uranium mineral pitchblende emitted significantly more radiation than would have corresponded to the uranium content. Marie and Pierre Curie discovered the new and radioactive elements polonium and radium in the pitchblende in 1898. They also recognized the element thorium as radioactive.
Ernest Rutherford (atomic nucleus)
Joseph John Thomson established in 1897 that the cathode rays observed in gas discharge tubes were light material particles and not ether waves. Thomson was able to determine the ratio e / m of charge and mass of the particles called “electrons” and found that it was independent of the cathode material, filling gas and other circumstances, i.e. that the electrons were apparently universal components of the atoms. In 1904 Thomson created the plum pudding model , according to which the electrons were embedded in a uniformly positively charged sphere.
When examining radioactive substances, different types of radiation could be distinguished: deflection in the magnetic field showed that the penetrating beta rays were negatively charged; Becquerel eventually identified them as electrons. Ernest Rutherford and Thomas Royds established in 1908 that the less penetrating alpha radiation was doubly positively charged helium ions.
Rutherford's scattering experiments, in which he bombarded metal foils with alpha particles, showed in 1911 that the positive charges of the atoms are concentrated in a small nucleus and the electrons are outside the nucleus - but their arrangement and number were still unknown.
Henry Moseley (ordinal number)
The analysis of his scattering experiments had Rutherford completed in 1911 to determine that the positive charge of the nucleus about corresponds to half the atomic mass: . Antonius van den Broek pointed out that the atomic mass increases by two units from one element to the next, that according to Rutherford's formula, the number of charges in the nucleus increases by one from one element to the next. The number of possible elements is therefore equal to the number of possible nuclear charges and each possible nuclear charge corresponds to a possible element. The atomic number also determines the position of each element in the periodic table. (The increase in atomic mass by two units is only a rough approximation; van den Broek was influenced here by his assumption that all atoms are made up of half alpha particles of mass number 2.)
Henry Moseley confirmed that the atomic number (also: atomic number) is a more suitable ordering principle for the elements than the atomic mass. He took advantage of the fact that materials bombarded with electrons emit not only the braking spectrum (Röntgen 1895) but also X-rays with a wavelength characteristic of the material ( Barkla , ca.1906 ) and that the wavelength of this radiation can be determined by means of diffraction on crystals ( von Laue 1912). Moseley determined the wavelengths of the characteristic radiation of various elements and found that the frequencies of these radiations were proportional to the square of an integer that described the position of the element in question in the periodic table ( Moseley's law ). He recognized this number as the number of charges in the nucleus. It was thus possible to easily determine the atomic number of an element experimentally.
Moseley demonstrated that many of the 70 or so allegedly newly discovered elements that competed for the 16 gaps to be filled in Mendeleev's periodic table could not exist because there was no space for them in the grid of ordinal numbers.
The good ten “ rare earths ” (their exact number was not known at the time) are difficult to separate chemically because they are very similar to one another. Mendeleev had found no place for them in his scheme. Moseley's ordinal number clearly assigned them the places 57 to 71.
The pioneers of the periodic table had to correct the occasional mass inversions (such as between iodine and tellurium) by swapping the relevant elements in the atomic mass scheme, without being able to give a reason for this, except that they fit better into the scheme of chemical similarities. Moseley's atomic number confirmed the correct order of the exchanged elements, the atomic masses had been misleading here.
When the First World War broke out , Moseley volunteered for military service and fell in the Battle of Gallipoli . Moseley's successors completed the systematic measurements and determined that uranium (the heaviest known element up to that point) has the atomic number 92, i.e. there are exactly 92 elements in the element series from hydrogen to uranium. Gaps were recognized in the ordinal numbers 43, 61, 72, 75, 85, 87 and 91, which could be filled in the following decades with the relevant new discoveries.
Frederick Soddy (isotopes)
In 1902, Ernest Rutherford and Frederick Soddy determined that the radioactive elements not only gave off radiation, but consisted of unstable atoms that spontaneously converted into new elements by giving off alpha, beta or gamma radiation - in obvious contradiction to what was previously assumed Indivisibility and immutability of atoms. Starting with actinium ( Debierne 1899), numerous other radioactive substances were quickly discovered after polonium and radon (in 1912 their number had grown to about 30). The new substances were initially viewed as separate elements and it seemed that the periodic table could not accommodate them all. It turned out, however, that almost all of them were chemically indistinguishable from elements already known and as such already had a place in the periodic table. For example, a decay product, initially called “radium D” , could not be distinguished from lead. On the other hand, exact determinations of the atomic mass showed that lead samples from different sources could have different atomic masses. Theodore William Richards found an atomic mass of 206.4 for lead made from pitchblende and 208.4 for lead made from thorite .
From several similar cases in 1911 Soddy drew the conclusion that one and the same element could be a mixture of different atomic masses. In 1913 he coined the term “isotopes” for atoms with the same atomic number but different mass numbers. Soddy and Kasimir Fajans set up the displacement theorems , according to which an atom of a given element loses two nuclear charges when an alpha particle is emitted and is transferred to the element that is two places further to the left in the periodic table, while it is one when a beta particle is emitted Place further to the right. For the elements between lead and uranium, it was obvious why they could exist with different atomic masses. A thorium atom (atomic number 90) can, for example, have arisen from uranium-235 via an alpha decay and then has the mass 231. It can also have arisen from actinium-230 via a beta decay and then has the mass 230. Soddy was able to use it for Predict lead from uranium ores the mass number 206 and for lead from thorium ore the mass number 208, even before Richards presented the results of his measurements.
In the 1920s, the large number of newly discovered isotopes plunged the periodic table into a crisis, as it seemed that instead of the elements, the much larger number of isotopes with their different atomic masses had to be brought into a systematic order. Fritz Paneth and George de Hevesy showed, however, that the chemical properties of the isotopes of an element were practically identical, so that it was justified to regard them as the same element according to their common atomic number (as a new ordering criterion instead of atomic mass) and thus to keep the periodic table .
Francis William Aston developed the first mass spectrograph in 1919 and found that the other elements that did not come from decay series could also be a mixture of different isotopes. This clarified why the (mean) atomic masses of some elements, such as chlorine, deviated so significantly from the integer. And that iodine, for example, has a higher atomic number but a smaller atomic mass than tellurium, was understandable as a consequence of the respective terrestrial isotope mixtures of the two elements.
Niels Bohr (construction principle)
When an excited atom emits the excitation energy again, the emitted radiation usually has a precisely defined wavelength that depends on the type of atom and the excited state. Rutherford's atomic model, in which the electrons moved around a central nucleus, made it clear why there was radiation at all, since the electrons had to send out electromagnetic waves as moving charges. However, it could not explain why only certain wavelengths were emitted. In addition, the electrons, which are in constant motion, would have had to constantly emit energy and, because of this continuous loss of energy, would have had to fall into the nucleus in the shortest possible time.
Niels Bohr took up Max Planck's discovery that the energy distribution of black body radiation can only be explained on the assumption that the energy radiation does not take place continuously but in the form of discrete “energy packets”. In 1913 he created an atomic model of hydrogen in which an electron orbits the nucleus not on any, but on one of several permitted orbits, whereby - according to Bohr's postulate , which is not substantiated - it does not emit any energy. The electron is more energetic on the higher orbits. If it falls back on a deeper orbit, it gives off the energy difference in the form of radiation. The frequency of this radiation is not (as would have been expected according to Maxwell's electrodynamics) the oscillation frequency of the orbiting electron, but rather, according to a further postulate of Bohr, given by Planck's condition with Planck's constant . Bohr succeeded in formulating the condition for the permitted orbits in such a way that the energy differences between every two orbits exactly corresponded to the observed frequencies of the spectral lines in the spectrum of hydrogen . The Bohr model of the atom was thus able to describe the hydrogen spectrum successfully; it also provided good results for the spectral lines of other atoms with only one electron (H, He + , Li ++ etc.).
Bohr also tried to describe the electron configurations of atoms with several electrons by distributing the electrons to the different orbits of his atomic model. His “construction principle” assumed that the electron configuration of an element could be derived from the configuration of the previous element by adding another electron (mostly on the outermost orbit). When a lane (a “bowl” in today's parlance) was full, the next lane began to be filled. However, Bohr could not deduce from his model how many electrons a path could take up and distributed the electrons, as was suggested by chemical and spectroscopic aspects.
Irving Langmuir (valence electron octet)
Gilbert Newton Lewis formulated the octet theory of the chemical bond in 1916 on the basis of the wealth of individual facts about the chemical and crystallographic behavior of substances, which has now accumulated . According to this theory, the atoms always strive for an octet of valence electrons as a particularly stable configuration and, in the case of non-ionic bonds, can reach this state by entering into a chemical bond with other atoms and completing their own octet with the valence electrons of these other atoms. Lewis called such a bond mediated by shared electrons a covalent bond . He imagined the valence electrons to be arranged at the eight corners of a cube surrounding the nucleus.
Irving Langmuir arranged the octets in bowls, the diameter of which corresponded to the orbits in Bohr's atomic model. He was able to explain the individual behavior of the chemical elements using the octet rule: The noble gases already have a complete valence electron octet and are not inclined to form chemical bonds. Elements with one or a few electrons in the valence shell tend to give up these electrons. Elements that are missing one or a few electrons to complete an octet tend to take up the missing number of electrons. Langmuir was even able to explain the different values of the elements, i.e. their tendency to combine with a certain number of the respective partner atoms ( Edward Frankland had introduced the concept in 1852). According to Langmuir, the valence is the number of electrons taken in or released to complete an octet. Chlorine, for example, accepts an electron, is thus monovalent and therefore combines with exactly one hydrogen atom, for example. (The current term of valence is kept more general.) Langmuir was also able to make the nature of isotopes understandable: Since the chemical behavior is determined by the valence electrons, all isotopes of an element obviously have the same number of valence electrons and thus the same number of valence electrons which is characteristic of this element chemical behavior. Different numbers of particles in the core, on the other hand, result in different atomic masses, but do not influence the chemical behavior.
Wolfgang Pauli (exclusion principle)
In 1921 Bohr took up the problem of the shell configuration of multi-electron atoms again. Arnold Sommerfeld had expanded Bohr's atomic model to include elliptical orbits and introduced a second quantum number to describe them. Bohr set up a new table with electron configurations in which a certain number of Sommerfeld's quantum numbers (in today's parlance: the secondary quantum numbers) was allowed for each orbit number (in today's parlance: each main quantum number).
Edmund Clifton Stoner created a table of shell configurations in 1924, in which he used a third quantum number, meanwhile introduced by Sommerfeld, to count the possible electron states. He was able to reproduce the values of the elements better than Bohr. The problem remained, however, that the number of additional spectral lines into which a spectral line splits when the atom is brought into a magnetic field suggests twice as many possible states of the electrons than had previously been considered. Wolfgang Pauli was able to explain Stoner's table by introducing a fourth quantum number (the "spin quantum number"), which can take on two different values and thus doubles the number of possible states, and by assuming that there are no two electrons in the atomic shell in all four quantum numbers could agree ( Paul's principle of exclusion ). This provided the reason why the main shells can each hold 2, 8, 18, 32 etc. electrons.
Hund's rule , established by Friedrich Hund in 1927, describes the order in which the individual orbitals of a subshell are filled with electrons: If several orbitals have the same energy level, they are first filled with individual electrons (with mutually parallel spins). Only then are the orbitals each occupied with a second electron (according to the Pauli principle with opposite spin).
Erwin Schrödinger (hydrogen problem)
Quantum mechanics was developed in the 1920s , beginning with de Broglie (1924) Heisenberg (1925) and Schrödinger (1926). It replaced the descriptive electron trajectories of Bohr's model of the atom with abstract, mathematically described "orbitals" .
In the case of the simple hydrogen atom, the quantum mechanical Schrödinger equation can be solved exactly. There is not just a single solution for this so-called “ hydrogen problem ”, but a whole set of solution functions that describe the various possible states of the electron. It is a set of discrete , that is, individually countable mathematical functions, which can therefore be differentiated from one another by "code numbers". As it turns out, exactly four such indicators are necessary for the unambiguous identification of each state, which can be identified with the four quantum numbers already derived from the experiments. The relationship between the first three quantum numbers can be derived from the Schrödinger equation for the hydrogen atom:
- Starting with 1, the principal quantum number can take on any integer value:
- The secondary quantum number can assume the following integer values, depending on what is present :
- The magnetic quantum number can assume the following integer values, depending on what is present :
According to Pauli's requirement, the spin quantum number can assume one of two possible values:
This now also provides a physical-mathematical justification for the number of possible electron states for a given main quantum number, i.e. for the maximum number of electrons that each main shell can hold.
In the main shell with, for example, there are 3 subshells that are differentiated by the secondary quantum numbers:
- The lower shell with contains 1 orbital with .
- The lower shell with contains 3 orbitals with .
- The lower shell with contains 5 orbitals with .
In total, this main shell contains 9 orbitals. Each orbital can accommodate two electrons , so that the main shell can contain a maximum of 18 electrons.
If one adds up the possible numbers of subshells and orbitals, one finds that a main shell with the main quantum number can hold electrons in total , i.e. for the electrons already known .
In atoms with several electrons, the electrons do not take on the one-electron states of the hydrogen atom just described, but rather multiple-electron states for which the quantum numbers just described are no longer valid. But they have analog quantum numbers for which the same designations are used.
Glenn T. Seaborg (Transuranium Elements)
Rutherford discovered in 1919 that nitrogen bombarded with alpha particles emitted a new type of particle. Together with James Chadwick , he identified these particles as positively charged hydrogen nuclei and called them “ protons ”. This identified the source of the positive charge on the atomic nucleus. Blackett and Harkins observed in a cloud chamber in 1925 that in such cases the alpha particle was swallowed instead of just knocking a proton out of the nitrogen nucleus in passing. It was concluded that according to the equation
the nitrogen atom had become an oxygen atom, the first example of an artificial element conversion (" transmutation "). Because of their lower charge, protons can overcome the electrical repulsion of heavier nuclei more easily than alpha particles and are therefore better suited as projectiles in bombardment experiments, since they can reach these nuclei more easily. But since there were no natural sources for protons with the required energy, proton accelerators were developed, partly as linear accelerators, but especially in the form of the cyclotron ( Lawrence and Livingston , 1931), which made numerous new transmutations possible.
When bombarded with alpha particles, beryllium, boron and lithium emitted a previously unknown, very penetrating radiation that Chadwick identified as uncharged particles with the mass of a proton. This “neutron” explained why different isotopes of an element could have the same atomic number but different masses: They had different numbers of neutrons in their nucleus. Since it is not repelled by the nuclei as an uncharged particle, the neutron is also suitable as a projectile in bombardment experiments.
In the course of the bombardment experiments it was possible to produce new, non-naturally occurring isotopes by transmutation (the first in 1934 was the phosphorus isotope with a mass number of 30 by Irène and Frédéric Joliot-Curie ). The artificially produced isotopes are radioactive ("artificial radioactivity") and, because of their specific manufacturability, were quickly used for scientific and practical purposes. The bombardment of uranium atoms with neutrons led to the discovery of nuclear fission in 1938 .
In 1940 , Edwin Mattison McMillan and Philip Hauge Abelson identified the new element neptunium in the products produced by bombarding uranium with neutrons . With the ordinal number 93 it was the first Transuran . A working group led by Glenn T. Seaborg examined the chemical properties of the new element (45 micrograms of it could be produced in a cyclotron). It was to be expected that a beta decay of the isotope neptunium-239 would lead to the formation of the element with atomic number 94. Targeted production of this isotope by bombarding uranium-238 with neutrons allowed Seaborg and colleagues to produce the new element, plutonium, in 1941.
I. II III IV V VI VII VIII ... 5 Rb Sr Y Zr Nb Mon Tc Ru ... 6th Cs Ba SE Hf Ta W. re Os ... 7th Fr. Ra Ac ThPaU... SE = La Ce Pr Nd Pm Sm ... 5 Rb Sr Y Zr Nb Mon Tc Ru ... 6th Cs Ba LAN. Hf Ta W. re Os ... 7th Fr. Ra ACT. Rf Db Sg Bra Hs ... LAN. = La Ce Pr Nd Pm Sm ... ACT. = Ac Th Pa U Np Pooh ...
Until then it was established that the rare earths ("SE" in the periodic table opposite) represent an insert in the sixth period of the periodic table, but in the seventh period the possible existence of a similar insert had not yet been recognized. Francium, radium and actinium clearly belonged to the first, second and third groups of the seventh period. It was assumed that the following elements thorium, protactinium and uranium had to belong to the fourth, fifth and sixth group, i.e. in the periodic table they would come under the transition metals hafnium, tantalum and tungsten. Some similarities with these groups, such as the value 4 of thorium or the value 6 of uranium, seemed to confirm the classification. This error delayed the progress of research, because the identification and separation of new elements often made use of their chemical similarity to known elements. The new substances were usually produced in too small quantities to be able to isolate them. However, if they were allowed to take part in a chemical reaction together with a known element, the product of which was then precipitated , and if the easily measurable radioactivity was found in the precipitate, then the chemical similarity to the known element was shown. (Marie and Pierre Curie had used this technique to concentrate the discovered radium in a barium chloride precipitate.) However, if the radioactivity remained in the solution, the presumed similarity was refuted. Incorrectly assumed chemical similarities had delayed work on protactinium and uranium, among other things. Seaborg recognized in 1944 that the newly created transuranic elements were not transition metals, but belonged to an insert in the seventh period (the actinides), which corresponds to the (now called lanthanoids) insert in the sixth period.
Seaborg and co-workers produced element 96 (curium) in 1944 by bombarding plutonium-239 with helium ions and shortly afterwards element 95 (americium) by bombarding plutonium-239 with neutrons. The bombardment of americium-241 with helium ions produced element 97 (Berkelium) in 1949, followed by element 98 (californium) by bombarding curium-242 with helium ions.
Several working groups identified the elements 99 (Einsteinium) and 100 (Fermium) in the fallout of the nuclear weapons test "Mike" (1952). With ever larger accelerators, heavier and heavier atoms could be used as projectiles, so that the production of ever heavier transuranic elements was also possible. The heaviest Transuran produced so far (as of 2019) is element 118 (Oganesson).
Ongoing discussions about positioning
Even today there are discussions about the position of some elements in the periodic table.
Classification of the first period
Because of the electron configuration, but not because of the elemental properties, helium (electron configuration 1s 2 ) would have to be classified in the second main group, i.e. above beryllium in the periodic table. Helium has only two electrons, in contrast to the other noble gases with eight electrons in the outermost shell. Since helium behaves chemically like a noble gas, it is in the eighth main group with the other noble gases. When the noble gases were discovered around 1900, they were assigned to the zeroth main group, which no longer exists today. At that time, helium was at the top (i.e. in the first period) of the zeroth main group. Today the noble gases are positioned in the eighth main group according to IUPAC.
Hydrogen can be positioned more clearly in the periodic table compared to helium, because it can assume the oxidation numbers 0 and +1 typical for the first main group and, like the lithium below, can enter into covalent bonds and is therefore counted among the alkali metals, even if it is the only gaseous one Is alkali metal and has a comparatively high electronegativity. Hydrogen forms alloy-like metal hydrides with some transition metals. Nevertheless, due to its non-metallic chemical reactivity, hydrogen is occasionally classified in the seventh main group with the halogens. Hydrogen is therefore listed twice in some periodic tables, in the first and seventh main group, albeit rarely. It has also been proposed to sort hydrogen above carbon because its electronegativity, electron affinity and ionization potential are closer to carbon, even if it can only react with one electron, in contrast to the representatives of the carbon group (fourth main group), which has four Electrons can react.
In order to take into account the different properties of hydrogen and helium, both are shown outside of the periodic table in rare cases.
Lanthanoids and actinides
The classification of the lanthanides and actinides is relatively different compared to elements from other periods. Early attempts placed the lanthanoids and actinides between the main group elements. Bohuslav Brauner sorted the lanthanides and actinides below zirconium in 1902 - this arrangement was referred to as the "asteroid hypothesis" based on several asteroids in the same orbit, which Brauner described in a letter to Mendeleev in 1881. In 1922, Niels Bohr placed the lanthanoids and actinides between the s block and the d block. A periodic table was developed by Glenn T. Seaborg between 1944 and 1949, which shows the lanthanoids and actinides as footnotes below yttrium. However, it was also criticized that such a classification tears apart the representation of the periodic table.
Scandium and yttrium are now relatively fixed, but the elements in the first subgroup below vary. Below yttrium, depending on the representation, there are either the first representatives of the lanthanides and actinides (lanthanum and actinium, i.e. in the order Sc-Y-La-Ac), or more rarely the last representatives of the lanthanides and actinides (lutetium and lawrencium, i.e. in the order Sc-Y-Lu-Lr) or a gap with footnotes (i.e. in the order Sc-Y - * - *). These three variants are based on the discussion of where the f block begins and ends. In a fourth variant, the third group is interrupted and an actinide-lanthanoid branch and a lutetium-lawrencium branch are inserted. There are chemical and physical arguments for the variant with Lawrencium and Lutetium below yttrium, but this variant does not find a majority among experts on the subject of the periodic table. Most chemists are unfamiliar with this discussion. In 2015, the IUPAC set up a project group on the arrangement of the lanthanides and actinides, which is to issue a recommendation - in 2019 there is no such recommendation.
Periodic table after discoverers of the elements
The dating of the discovery of such chemical elements, which have been known since prehistoric times or antiquity , is only imprecise and, depending on the literature source, can vary by several centuries. More reliable dating is only possible from the 18th century. Until then, only 15 elements were known and described as such: 12 metals (iron, copper, lead, bismuth, arsenic, zinc, tin, antimony, platinum, silver, mercury and gold) and three non-metals (carbon, sulfur and phosphorus). Most of the elements were discovered and scientifically described in the 19th century. At the beginning of the 20th century only ten of the natural elements were unknown. Since then, mainly difficult to access, often radioactive elements have been shown . Many of these elements do not occur naturally and are the product of artificial nuclear fusion processes . It was not until December 1994 that the two artificial elements Darmstadtium ( Eka -Platinum) and Roentgenium (Eka-Gold) were produced. Until the element names have been determined, new elements are identified with systematic element names .
This periodic table gives an overview of the discoverers or producers of the individual elements by clicking on the element identifier. For the elements for which no discoverer / producer is known, the current historical state of knowledge is shown briefly under the overview plan.
| group | 1 | 2 | 3 | 4th | 5 | 6th | 7th | 8th | 9 | 10 | 11 | 12 | 13 | 14th | 15th | 16 | 17th | 18th | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| period | ||||||||||||||||||||
| 1 |
H + |
He + |
||||||||||||||||||
| 2 |
Li + |
Be + |
B + |
C + |
N + |
O + |
F + |
Ne + |
||||||||||||
| 3 |
Na + |
Mg + |
Al + |
Si + |
P + |
S + |
Cl + |
Ar + |
||||||||||||
| 4th |
K + |
Ca + |
Sc + |
Ti + |
V + |
Cr + |
Mn + |
Fe + |
Co + |
Ni + |
Cu + |
Zn + |
Ga + |
Ge + |
As + |
Se + |
Br + |
Kr + |
||
| 5 |
Rb + |
Sr + |
Y + |
Zr + |
Nb + |
Mon + |
Tc + |
Ru + |
Rh + |
Pd + |
Ag + |
C d + |
In + |
Sn + |
Sb + |
Te + |
I + |
Xe + |
||
| 6th |
Cs + |
Ba + |
* + |
H f + |
Ta + |
W + |
R e + |
Os + |
Ir + |
Pt + |
Au + |
Hg + |
T l + |
Pb + |
Bi + |
Po + |
At + |
Rn + |
||
| 7th |
Fri + |
Ra + |
** + |
Rf + |
D b + |
S g + |
B h + |
Hs + |
Mt + |
D s + |
Rg + |
C n + |
Nh + |
Fl + |
Mc + |
Lv + |
Ts + |
Og + |
||
| * |
La + |
C e + |
Pr + |
Nd + |
Pm + |
Sm + |
Eu + |
Gd + |
Tb + |
Dy + |
Ho + |
He + |
Tm + |
Yb + |
Lu + |
|||||
| ** |
Ac + |
Th + |
Pa + |
U + |
Np + |
Pu + |
On + |
Cm + |
Bk + |
C f + |
E s + |
F m + |
M d + |
No + |
Lr + |
|||||
| before 1800 | 1800-1849 | 1850-1899 | 1900-1949 | 1950-1999 | since 2000 |
|
|||||
2019: International Year of the Periodic Table
The United Nations (UN) has declared 2019 the "International Year of the Periodic Table of the Chemical Elements" (IYPT 2019): With this, they want to arouse awareness worldwide of how chemistry promotes sustainable development and solutions to global challenges in energy , education , agriculture or Health can offer. In this way, the most recent discoveries and names of four "super-heavy" elements of the periodic table with the ordinal numbers 113 (Nihonium), 115 (Moscovium), 117 (Tenness) and 118 (Oganesson) should be made better known. The dedication also coincides with the 150th anniversary of the development of the periodic table. Events in Paris , Murcia and Tokyo will commemorate the event.
Future extensions of the periodic table
Experiments to create synthetic elements continue and are expected to result in the creation of elements with atomic numbers above 118 as well. If these fit into the previous scheme, a g -subshell (namely that of the fifth main shell) is filled for the first time in the eighth period . Since the g subshell contains nine orbitals that can hold 18 electrons, the eighth period will comprise a total of fifty (2 · 5 2 ) elements: eight main group elements (filling the 8s and 8p subshells ), ten outer transition elements (filling the 7d lower shell), fourteen inner transition elements in which the 6f lower shell is filled up, and a further eighteen inner transition elements in which the 5g lower shell is filled up. The same would apply to the ninth period.
It is possible that the relativistic effects that increase with the ordinal number ( see above ) will blur the periodicities more and more. They influence the behavior of the electrons and thus the chemical properties so that they no longer necessarily have to correspond to the position of the element in the periodic table. This is already indicated by the known elements: The transition metals 104 rutherfordium and 105 dubnium should be similar in their behavior to the transition metals hafnium and tantalum, respectively, which are above in the periodic table. However, experiments show a behavior that is more similar to the actinides plutonium or protactinium. The following elements 106 Seaborgium and 107 Bohrium, however, again show the behavior corresponding to their position in the periodic table. 114 Flerovium should resemble lead as an element of the fourth main group, but it seems to behave more like a precious metal.
In general, the heavier the atoms generated, the shorter their lifespan. Theoretical estimates suggest that from ordinal numbers of a little over 170 the lifetime of the generated atoms approaches zero, so that one can no longer speak of generated atoms at all. If applicable, this would be the theoretical upper limit for the scope of the periodic table.
Other representations of the periodic table
Long period system
The medium-length form of the periodic table (with 18 columns and a space-saving f- block) has already been explained in detail. If you do not move the f -block, which includes the lanthanides and actinides, you get the so-called long form of the periodic table with 32 columns. In this representation, in contrast to the medium-long form, there are no interruptions in the sequence of the ordinal numbers.
A first long-period system was proposed by Alfred Werner in 1905 . William B. Jensen recommended the long period table because the lanthanoids and actinides shown separately in shorter period tables would seem unimportant and boring to students. Despite the seamless display, the long-period system is rarely used because of its unwieldy format for book printing. A periodic table that goes beyond the atomic number 118 can be found under Extended Periodic Table .
Alternative periodic tables
The form of the periodic table by Dmitri Mendeleev has prevailed. Nevertheless, there were (and are) other suggestions for alternative arrangements of the elements according to their properties. In the first hundred years since Mendeleev's draft of 1869, an estimated 700 variants of the periodic table were published. In addition to many rectangular variants, there were also circular, spherical, cube, cylinder, spiral, pyramidal, layered, flower, loop, octagonal and triangular periodic tables. The different shapes mostly serve to emphasize certain properties. Most representations are two-dimensional . The first three-dimensional representation was published by de Chancourtois in 1862 before Mendeleev's periodic table. Another three-dimensional representation of several loops of paper was published by M. Courtines in 1925, and a layered representation was made by AN Wrigley in 1949. Paul-Antoine Giguère published a periodic table compiled from several plates in 1965 and Fernando Dufour a tree-shaped representation in 1996. Tim Stowe's periodic table from 1989 was described as four-dimensional, including one color dimension.
In addition, there are more chemically and more physically oriented representations of the periodic table. A chemically oriented periodic table was published in 2002 by Geoff Rayner-Canham for inorganic chemists, emphasizing tendencies and patterns as well as unusual chemical properties. A physically oriented periodic table was published by Charles Janet in 1928 , with a stronger focus on electron configuration and quantum mechanics, as was also done by Alper in 2010. The latter, however, was criticized due to the lack of representation of the periodicity of the properties. The mixed forms include the standard periodic system, which lists both chemical and physical properties such as oxidation numbers, electrical and thermal conductivity. Its prevalence is attributed to the balance and practicality of the properties displayed. The short periodic system ( see above ), in which main and subgroups are nested in one another , is not an alternative periodic table, but a clearly different representation . Other classification methods are based on the natural occurrence of the elements in minerals ( Goldschmidt classification ) or on the crystal structure .
Quote
“In addition to predicting new elements and their expected properties, the periodic table has also proven to be invaluable when looking for promising research approaches in the production of new compounds. Chemists have internalized this way of thinking to such an extent that they are barely aware of how extremely difficult their task would be if they could not rely on periodic trends. Your work can be planned successfully because the effects of replacing an element or group in a compound can be assessed in advance. The prudent chemist always keeps an eye on the possibility that surprisingly new effects or unexpected factors may occur. "
See also
literature
- Ekkhard Fluck, Klaus G. Heumann: Periodic table of the elements: physical properties; [chemical, biological and geological properties]. 5th edition, Wiley-VCH, Weinheim 2012, ISBN 978-3-527-33285-4 .
- Periodic table interactive! (CD-ROM for Windows and Mac OS X), Welsch & Partner, Tübingen.
- P. Kurzweil, P. Scheipers: Chemistry. Vieweg + Teubner, Wiesbaden 2010, ISBN 978-3-8348-0341-2 , Chapter 3: Periodic Table of the Elements (PSE) .
- NN Greenwood, A. Earnshaw: Chemistry of the Elements. 2nd ed., Elsevier, Oxford 2016, ISBN 978-0-7506-3365-9 , Chapter 2: Chemical Periodicity and the Periodic Table .
- K. Seubert (Ed.): The natural system of the chemical elements - treatises by Lothar Meyer (1864–1869) and D. Mendelejeff (1869–1871). Engelmann, Leipzig 1895 ( digitized version ).
- Stephen G. Brush : The reception of Mendeleev's Periodic Law in America and Britain. In: Isis. Volume 87, 1996, pp. 595-628.
- Jan W. van Spronsen : The Periodic System of Chemical Elements. A History of the First Hundred Years. Elsevier, Amsterdam, London and New York 1969, ISBN 978-0-444-40776-4 .
- Masanori Kaji, Helge Kragh , Gábor Palló (Eds.): Early responses to the periodic system. Oxford University Press, 2015, ISBN 978-0-19-020007-7 .
- Eric Scerri : The Periodic Table. Its story and its significance. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 .
Web links
- Thomas Seilnacht: Periodic Table for Chemistry Classes
- Entry on the periodic table, Helmut Sitzmann (editor). In: Römpp Online . Georg Thieme Verlag, accessed on June 4, 2020.
- Spectrum of Science / Society of German Chemists: Elements, 150 Years of the Periodic Table , 2019 (pdf)
- web element - information on the elements (English)
- Periodic Table , by Theodore Gray
- PDF print version at pse-online.de (135 kB)
- Setting the table. A brief visual history of the periodic table. Animation on sciencemag.org , last viewed on February 1, 2019
- Ralph M. Cahn: Historical and Philosophical Aspects of the Periodic Table of the Chemical Elements (PDF; 560 kB)
- Eric Scerri, Mendeleev's Legacy: The Periodic System , Distillations, April 12, 2007
- Internet Database of Periodic Tables , an extensive collection of historical and modern periodic tables
- Periodic table of the European chemicals agency Echa - provides chemical and physical properties as well as a lot of regulatory and legal information that is otherwise difficult to find
Individual evidence
- ^ Wilhelm Pape , Max Sengebusch (arrangement): Concise dictionary of the Greek language . 3rd edition, 6th impression. Vieweg & Sohn, Braunschweig 1914 ( images.zeno.org [accessed on March 6, 2019]).
- ↑ Heinz F Wendt: Langenscheidts pocket dictionary of the modern Greek and German language . 13th edition. Langenscheidt, Berlin 1986.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 16.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 10.
- ↑ Basics and main group elements. in: Hollemann, Wiberg, Textbook of Inorganic Chemistry , Walter de Gruyter, 2016, ISBN 978-3-11-049585-0 , Volume 1, p. 82.
- ^ Chemistry: Four elements added to periodic table. (No longer available online.) In: BBC News. January 4, 2016, archived from the original on January 4, 2016 ; accessed on September 12, 2018 (English).
- ^ Nicholas St. Fleur: Four New Names Officially Added to the Periodic Table of Elements. (No longer available online.) In: New York Times. December 1, 2016, archived from the original on August 14, 2017 ; accessed on September 12, 2018 (English).
- ↑ a b Winfried Koelzer: Lexicon on nuclear energy. KIT Scientific Publishing, 2013, ISBN 978-3-7315-0059-9 , p. 50.
- ^ A b c J. Emsley: Nature's Building Blocks: An AZ Guide to the Elements , New. Edition, Oxford University Press, New York, NY 2011, ISBN 978-0-19-960563-7 .
- ^ Discovery and Assignment of Elements with Atomic Numbers 113, 115, 117 and 118 - IUPAC - International Union of Pure and Applied Chemistry. In: iupac.org. June 8, 2016, accessed March 15, 2019 .
- ↑ Hans Peter Latscha, Martin Mutz: Chemistry of the elements. ISBN 3-642-16914-7 , p. 1.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 96.
- ^ A b c A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 99.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 101.
- ^ A b c A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 102.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 97.
- ↑ a b A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , pp. 100-101.
- ^ A b c d e f A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , pp. 327–341.
- ^ A b c d e A. F. Holleman , N. Wiberg : Inorganic Chemistry . 103rd edition. Volume 2: Subgroup elements, lanthanoids, actinides, transactinides. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049590-4 , pp. 1537-1540.
- ↑ L. Pauling: General Chemistry. Dover Publications, New York 1988, ISBN 978-0-486-65622-9 , p. 621.
- ↑ L. Pauling: General Chemistry. Dover Publications, New York 1988, ISBN 978-0-486-65622-9 , p. 133.
- ↑ a b c d e f L. Pauling: General Chemistry. Dover Publications, New York 1988, ISBN 978-0-486-65622-9 , p. 172.
- ^ A b c A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 81.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 2: Subgroup elements, lanthanoids, actinides, transactinides. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049590-4 , pp. 2227–2231.
- ↑ a b c d e f g h i j k l m n o A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , pp. 330-340.
- ↑ a b A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 149.
- ↑ a b A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 157.
- ↑ a b c d e f g L. Pauling: General Chemistry. Dover Publications, New York 1988, ISBN 978-0-486-65622-9 , p. 183.
- ↑ a b R. Karsten: Construction Chemistry: Handbook for Study and Practice. 9th edition, Müller, Karlsruhe 1992, ISBN 3-7880-7438-8 , p. 43.
- ^ R. Karsten: Construction chemistry: Handbook for study and practice. 9th edition, Müller, Karlsruhe 1992, ISBN 3-7880-7438-8 , p. 44.
- ↑ a b c NN Greenwood, A. Earnshaw: Chemistry of the Elements. 2nd ed., Elsevier, Oxford 2016, ISBN 978-0-7506-3365-9 , p. 27.
- ↑ a b c NN Greenwood, A. Earnshaw: Chemistry of the Elements. 2nd ed., Elsevier, Oxford 2016, ISBN 978-0-7506-3365-9 , p. 65.
- ↑ L. Pauling: General Chemistry. Dover Publications, New York 1988, ISBN 978-0-486-65622-9 , p. 624.
- ↑ L. Pauling: General Chemistry. Dover Publications, New York 1988, ISBN 978-0-486-65622-9 , p. 625.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 18.
- ↑ a b c R. Karsten: Construction chemistry: Handbook for study and practice. 9th edition, Müller, Karlsruhe 1992, ISBN 3-7880-7438-8 , p. 78f.
- ↑ a b R. Karsten: Construction Chemistry: Handbook for Study and Practice. 9th edition, Müller, Karlsruhe 1992, ISBN 3-7880-7438-8 , p. 78.
- ↑ a b c Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 14.
- ^ R. Karsten: Construction chemistry: Handbook for study and practice. 9th edition, Müller, Karlsruhe 1992, ISBN 3-7880-7438-8 , p. 79.
- ^ R. Karsten: Construction chemistry: Handbook for study and practice. 9th edition, Müller, Karlsruhe 1992, ISBN 3-7880-7438-8 , p. 81.
- ^ R. Karsten: Construction chemistry: Handbook for study and practice. 9th edition, Müller, Karlsruhe 1992, ISBN 3-7880-7438-8 , p. 82.
- ^ A b NN Greenwood, A. Earnshaw: Chemistry of the Elements. 2nd ed., Elsevier, Oxford 2016, ISBN 978-0-7506-3365-9 , p. 26.
- ^ GD Hinrichs: On the classification and the atomic weights of the so-called chemical elements, with particular reference to Stas's determinations Archived from the original on August 2, 2016. In: Proceedings of the American Association for the Advancement of Science . 18, No. 5, 1869, pp. 112-124.
- ^ A b c A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 339.
- ↑ a b c d e f Eric R. Scerri: The Periodic Table. Oxford University Press, USA, 2007, ISBN 978-0-19-530573-9 , pp. 272-276.
- ↑ a b Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , pp. 265-266.
- ^ G. Rayner-Canham: Isodiagonality in the periodic table. Foundations of Chemistry, vol. 13, issue 2 (July 2011), 121-129, doi: 10.1007 / s10698-011-9108-y (Paywall)
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 654.
- ^ G. Rayner-Canham: Periodic Patterns: the Group (n) and Group (n + 10) linkage. Foundations of Chemistry, vol. 15, issue 2 (July 2013), 229-237, doi: 10.1007 / s10698-012-9169-6 (Paywall)
- ↑ a b c d Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , pp. 267 ff.
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 81–90. Edition. Walter de Gruyter, Berlin 1976, ISBN 3-11-005962-2 , pp. 748-750.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 291, footnote 26.
- ↑ a b c Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 11.
- ^ IUPAC: Nomenclature of Inorganic Chemistry - IUPAC Recommendations 2005. (“Red Book”) RSCPublishing, Cambridge 2005, ISBN 0-85404-438-8 ( PDF 4.3 MB ), p. 51; on the IUPAC website: Group 1-18 and collective names
- ↑ a b c d e f g Chemistry: cracks in the periodic table. In: Spektrum.de. Retrieved January 19, 2019 .
- ↑ Hans-Ulrich Harten: Physics for doctors. Springer-Verlag, 2013, ISBN 978-3-662-22293-5 , p. 77.
- ↑ Roland Lindner: Nuclear and Radiochemistry. Springer-Verlag, 1961, Reprint 2013, ISBN 978-3-642-87162-7 , p. 229.
- ↑ Chemistry: Island of the Heavyweights. In: Spektrum.de. Retrieved January 19, 2019 .
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 89.
- ^ A b c A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 71.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , pp. 72–73.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , plate 1.
- ^ A b c A. F. Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , Appendix III.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 479.
- ↑ a b c Entry on chemical elements. In: Römpp Online . Georg Thieme Verlag, accessed on July 10, 2019.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , pp. 78 f.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 91.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , pp. 91 ff.
- ↑ a b B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 94.
- ^ A b c B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , pp. 229-230.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 41.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , pp. 116 ff.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , pp. 63 ff.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 66.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 256.
- ^ Anonymus, Nachrichten aus Chemie und Technik. (1972), Vol. 20, pp. 459-460.
- ^ E. Renatus, In: Chemistry of Our Time (1983), Volume 17, pp. 96-102.
- ↑ a b c d e f g Entry on periodic table. In: Römpp Online . Georg Thieme Verlag, accessed on June 6, 2019.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 78.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 42.
- ↑ Excerpt from a letter from Hofrath Wurzer, Prof. der Chemie zu Marburg. in: LW Gilbert (Ed.): Annalen der Physik, New Series, Volume 26, Leipzig 1817, pp. 331–334, digitized digitized version .
- ↑ a b L. Gmelin: Handbook of theoretical chemistry. 1st volume, 1st department, 3rd edition, Frankfurt am Main 1827, pp. 35–36 ( digitized version ).
- ↑ JW Döbereiner: Attempt to group elementary substances according to their analogy. in: JC Poggendorff (Ed.): Annals of Physics and Chemistry, Volume 15, Leipzig 1829, pp. 301–307 Digitalisat Digitalisat
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 43.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 43, calculation or printing errors 80,470 corrected.
- ^ L. Gmelin: Handbook of theoretical chemistry. 1st volume, 1st department, 3rd edition, Frankfurt am Main 1827, p. 192 ( digitized version ).
- ^ L. Gmelin: Handbuch der Chemie. Volume 1, 4th edition, Heidelberg 1843, p. 457 ( digitized version ).
- ↑ a b c Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , pp. 55-56.
- ↑ a b E. Lenßen: On the grouping of the elements according to their chemical-physical character. Justus Liebig's Annals of Chemistry and Pharmacy, CIII. Volume, 2nd issue (1857), pp. 121-131, doi: 10.1002 / jlac.18571030202 .
- ↑ Ernst Lenssen: On the theory of colors. Justus Liebig's Annals of Chemistry and Pharmacy. CIV. Volume, 2nd issue (1857), pp. 177-184, doi: 10.1002 / jlac.18571040206 .
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 68.
- ↑ a b c d e f g h Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , pp. 76-77.
- ↑ a b c NN Greenwood, A. Earnshaw: Chemistry of the Elements. 2nd ed., Elsevier, Oxford 2016, ISBN 978-0-7506-3365-9 , p. 29.
- ↑ a b Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , pp. 68-69.
- ↑ Masanori Kaji, Helge Kragh, Gabor Pallo: Early Responses to the Periodic System. Oxford University Press, 2015, ISBN 978-0-19-020008-4 , pp. 50-57.
- ↑ a b c d Eric Scerri: Mendeleev's Legacy: The Periodic System. Science History Institute Distillations. Retrieved June 6, 2019.
- ↑ JW van Spronsen: The Periodic System of Chemical Elements: A History of the First Hundred Years . Elsevier, Amsterdam 1969.
- ↑ Julius Lothar Meyer: The modern theories of chemistry. Maruschke & Berendt, Breslau 1864, table on p. 137 .
- ↑ Julius Lothar Meyer: The modern theories of chemistry. Maruschke & Berendt, Breslau 1864, p. 138 .
- ↑ Julius Lothar Meyer: The modern theories of chemistry. Maruschke & Berendt, Breslau 1864, p. 136 .
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 94.
- ↑ a b c Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 96.
- ↑ a b L. Meyer: The nature of the chemical elements as a function of their atomic weights , Annalen der Chemie und Pharmacie, VII. Supplementary volume 1870, pp. 354-364, Google-Books Archive.org
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 98.
- ↑ Dmitri Ivanovich Mendeleev: An experiment for a system of elements based on their atomic weights and chemical similarities (translation), 1869.
- ↑ a b c Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 101.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 123.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 117.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 105.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 106.
-
↑ a b c d
D. Mendeleev, J. Russ. Phys. Chem. Soc., 1, 60 (1869).
German translation:
D. Mendelejeff: The relationships between the properties of the elements and their atomic weights. In: K. Seubert (ed.): The natural system of chemical elements - treatises by Lothar Meyer (1864–1869) and D. Mendelejeff (1869–1871). Engelmann, Leipzig 1895, p. 20 ( digitized version ). -
↑ a b c d
D. Mendelejeff: About the relations of the properties to the atomic weights of the elements. Journal for Chemistry, New Series, Volume V, Quandt & Handel, Leipzig 1869, p. 405 ( digitized version )
D. Mendelejeff: Ueber the relations of the properties to the atomic weights of the elements. In: K. Seubert (ed.): The natural system of chemical elements - treatises by Lothar Meyer (1864–1869) and D. Mendelejeff (1869–1871). Engelmann, Leipzig 1895, p. 18 ( digitized version ). - ^ Correspondences: V. von Richter, from St. Petersburg on October 17, 1869. Reports of the German Chemical Society, 2nd year, Ferd. Dümmler's Verlagsbuchhandlung, Berlin 1869, p. 552 ff., Here p. 553 ( digitized version ).
-
↑
D. Mendeleev, J. Russ. Phys. Chem. Soc., 3, 25 (1871).
German translation:
D. Mendelejeff: The periodic regularity of the chemical elements. Annals of Chemistry and Pharmacy, VIII. Supplementary volume, 133–229, CF Winter'sche Verlagshandlung, Leipzig and Heidelberg 1872 ( digitized version )
D. Mendelejeff: The periodic regularity of chemical elements. In: K. Seubert (ed.): The natural system of chemical elements - treatises by Lothar Meyer (1864–1869) and D. Mendelejeff (1869–1871). Engelmann, Leipzig 1895, p. 41 ( digitized version ). - ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 246, p. 253 and Fig. 9.7, p. 245.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 131 ff.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . S. 139. The heat capacities 0.073 and 0.076 given for Scerri without units are evidently in Btu / (lb ° F) and were converted into J / (kg K) by multiplying them by 4186.8.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 143.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 151.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 291, footnote 32.
- ↑ a b Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 156.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 112.
- ↑ In the poison cabinet: Oldest copy of the periodic table found. In: Spektrum.de. Retrieved January 19, 2019 .
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 302, footnote 47.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 161 f.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 186.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , pp. 488 f.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 487.
- ^ A b AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 482.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 500.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 490.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , pp. 211 f.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 164.
- ↑ a b c d Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 165 f.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 170.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 161.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 223.
- ^ The Nobel Prize - Charles Glover Barkla: Facts : "Around 1906, Charles Barkla showed that each element's secondary spectrum was unique, irrespective of temperature, structure, and chemical composition."
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 222.
- ↑ a b Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 171.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 172.
- ↑ a b c d B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 227.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 175.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 173 f.
- ^ A b c AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 493.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 492.
- ^ A b c AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 494.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 177.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 178.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 178 (the ordinal number 91 given by Scerri for thorium must correctly read 90).
- ^ The Nobel Prize - Francis W. Aston: Facts : "... Francis Aston developed the mass spectrograph in 1919 to map the different isotopes."
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 496.
- ↑ a b Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 188.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 503.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 504.
- ↑ a b c Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 190.
- ↑ a b B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 246.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 247.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 249.
- ^ B. Jaffe: Crucibles: The Story of Chemistry. 4th ed., Dover, New York 1976, ISBN 978-0-486-23342-0 , p. 250.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 192.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 197 ff.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 196.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 199.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 200.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 202.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 230.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 506.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 231.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 507.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 234.
- ↑ Eric R. Scerri: The Periodic Table. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 . P. 320, footnote 21.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 508.
- ↑ a b c d AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 509.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , pp. 510 f.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 512.
- ^ A b AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 513.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 514.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 519.
- ^ A b c AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 522.
- ↑ a b c d AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 594.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 523.
- ↑ Eric Scerri: A Tale of Seven Elements. Oxford University Press, New York 2013, ISBN 978-0-19-539131-2 , p. 67.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 515.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 488.
- ^ AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 595.
- ^ A b AJ Ihde: The Development of Modern Chemistry. Dover, New York 1984, ISBN 978-0-486-64235-2 , p. 596.
- ^ T. Gray: The Elements: A Visual Exploration of Every Known Atom in the Universe. Black Dog & Leventhal Publishers. ISBN 978-1-57912-814-2 , p. 12.
- ↑ Errol G. Lewars: Modeling Marvels: Computational Anticipation of Novel Molecules . Springer Science & Business Media, December 5, 2008, ISBN 978-1-4020-6973-4 , pp. 69-71. Archived from the original on May 19, 2016.
- ^ IUPAC: IUPAC Periodic Table of the Elements . In: iupac.org . IUPAC. May 1, 2013. Archived from the original on August 22, 2015. Retrieved September 20, 2015.
- ^ PA Cox: Inorganic Chemistry , 2nd. Edition, Bios Scientific, London 2004, ISBN 978-1-85996-289-3 , p. 149.
- ↑ G. Rayner-Canham, T. Overton: Descriptive inorganic chemistry , 4th. Edition, WH Freeman, New York January 1, 2006, ISBN 978-0-7167-8963-5 , p. 203.
- ^ P Wilson: Hydrogen adopts alkali metal position . In: Chemistry World ' . Royal Society of Chemistry. 2013.
- ↑ GM Bodner, LH Rickard, JN Spencer: Chemistry: Structure and Dynamics . John Wiley & Son, New York 1995, ISBN 978-0-471-14278-2 , p. 101.
- ↑ Eric Scerri: Some comments on the recently proposed periodic table featuring elements ordered by their subshells . In: Journal of Biological Physics and Chemistry (2012), Volume 12, Issue 2.
- ^ G. Seaborg: The chemical and radioactive properties of the heavy elements . In: Chemical English Newspaper . 23, No. 23, 1945, pp. 2190-2193. doi : 10.1021 / cen-v023n023.p2190 .
- ^ MW Cronyn: The Proper Place for Hydrogen in the Periodic Table . In: Journal of Chemical Education . 80, No. 8, August 2003, pp. 947-951. bibcode : 2003JChEd..80..947C . doi : 10.1021 / ed080p947 .
- ^ NN Greenwood, A. Earnshaw: Chemistry of the Elements. Oxford, Pergamon Press, 1984. ISBN 978-0-08-022057-4 .
- ^ A b P. Thyssen, K. Binnemans: Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis . In KA Gschneider Jr. (Ed.): Handbook on the Physics and Chemistry of the Rare Earths. Elsevier, Amsterdam, 2011, Volume 41, pp. 1-94.
- ^ GT Seaborg: Origin of the Actinide Concept . In: KA Gschneider Jr. (Ed.): Handbook on the Physics and Chemistry of the Rare Earths . Volume 18. Amsterdam, Elsevier, 1994, pp. 1-27.
- ↑ PJ Stewart: The Flyleaf Table: An Alternative . In: Journal of Chemical Education . 85, No. 11, 2008, p. 1490. bibcode : 2008JChEd..85.1490S . doi : 10.1021 / ed085p1490 .
- ↑ G. Hevesy: Redkie zemeli s tochki zreniya stroeniya atoma (Rare earths from the point of view of structure of atom). Quoted in Trifonov 1970, p. 188 ( Russian ). NKhTI, Leningrad 1929.
- ^ Silberberg: Chemistry: The molecular nature of matter and change , 4th. Edition, McGraw-Hill, New York 2006, ISBN 978-0-07-111658-9 , p. 536.
- ↑ P. Thyssen, K. Binnemans: Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis . In KA Gschneider Jr. (Ed.): Handbook on the Physics and Chemistry of the Rare Earths. Elsevier, Amsterdam 2011, Volume 41, pp. 80-81.
- ↑ J. Keeler, P. Wothers: Chemical Structure and Reactivity: An Integrated Approach . Oxford University, Oxford 2014, ISBN 978-0-19-960413-5 , p. 259.
- ^ E. Scerri : Mendeleev's Periodic Table Is Finally Completed and What To Do about Group 3? Archived from the original on July 5, 2017. In: Chemistry International . 34, No. 4, 2012. doi : 10.1515 / ci.2012.34.4.28 .
- ↑ D. Castelvecchi: Exotic atom struggles to find its place in the periodic table. Archived from the original on October 5, 2015. In: Nature . April 8, 2015. doi : 10.1038 / nature.2015.17275 .
- ^ The constitution of group 3 of the periodic table . IUPAC. 2015. Archived from the original on July 5, 2016. Retrieved on July 30, 2016.
-
↑ Badische Zeitung: The bottom line: When it stinks and cracks. In: Badische Zeitung . January 2, 2019, accessed January 21, 2019 . 2019 will be the International Year of the Periodic Table. Chemistry Associations Baden-Württemberg, January 12, 2018, accessed on January 21, 2019 .
- ↑ International Year of the Periodic Table [1]
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 81–90. Edition. Walter de Gruyter, Berlin 1976, ISBN 3-11-005962-2 , p. 994.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 81–90. Edition. Walter de Gruyter, Berlin 1976, ISBN 3-11-005962-2 , p. 998.
- ↑ Eric Scerri: A Tale of 7 Elements: Element 61 — Promethium . Oxford University Press (USA), 2013, ISBN 978-0-19-539131-2 , pp. 175-194 (190). Archived from the original on September 10, 2017: "... no interruptions in the sequence of increasing atomic numbers ..."
- ^ A b William B. Jensen: Classification, symmetry and the periodic table . In: Comp. & Maths. With Appls. . 12B, No. I / 2, 1986. Retrieved January 2017.
- ↑ MR Leach: Concerning electronegativity as a basic elemental property and why the periodic table is usually represented in its medium form . In: Foundations of Chemistry . 15, No. 1, 2012, pp. 13-29. doi : 10.1007 / s10698-012-9151-3 .
- ^ Finding Aid to Edward G. Mazurs Collection of Periodic Systems Images .
- ↑ a b Eric Scerri: The periodic table: Its story and its significance. Oxford University Press, New York 2007, ISBN 978-0-19-530573-9 , p. 20.
- ↑ Weird Words of Science: Lemniscate Elemental Landscapes . In: Fields of Science . fieldofscience.com. March 22, 2009. Archived from the original on March 4, 2016. Retrieved January 4, 2016.
- ^ The Internet database of periodic tables
- ^ Courtines, J. Chemical Education, Vol. 2, 1925, p. 107.
- ^ Mark R. Leach: 1925 Courtines' Periodic Classification . Archived from the original on May 16, 2016. Retrieved October 16, 2012.
- ^ TO Wrigley, WC Mast, TP McCutcheon: A laminar form of the periodic table. Part I., J. Chem. Education, Volume 26, 1949, p. 216, doi : 10.1021 / ed026p216 . The name is misrepresented in van Spronsen, The Periodic System of Chemical Elements (1969).
- ^ Mark R. Leach: 1949 Wringley's Lamina System . Archived from the original on December 3, 2011. Retrieved October 16, 2012.
- ^ EG Mazurs: Graphical Representations of the Periodic System During One Hundred Years . University of Alabama Press, Alabama 1974, ISBN 978-0-8173-3200-6 , p. 111.
- ^ Mark R. Leach: 1996 Dufour's Periodic Tree . Archived from the original on April 18, 2010. Retrieved October 16, 2012.
- ↑ GB Kauffman: Elementree: A 3-D Periodic Table by Fernando Dufour, The Chemical Educator, Volume 4, 1999, pp 121-122.
- ^ Mark R. Leach: 1989 Physicist's Periodic Table by Timothy Stowe . Archived from the original on June 5, 2012. Retrieved October 16, 2012.
- ^ D. Bradley: At last, a definitive periodic table? Archived from the original on May 1, 2013. In: ChemViews Magazine . July 20, 2011. doi : 10.1002 / chemv.201000107 .
- ↑ Eric Scerri: The periodic table: Its story and its significance. Oxford University Press, New York 2007. ISBN 978-0-19-530573-9 , pp. 285-286.
- ↑ Mark R. Leach: 2002 Inorganic Chemist's Periodic Table . Archived from the original on March 9, 2013. Retrieved October 16, 2012.
- ^ E. Scerri : The role of triads in the evolution of the periodic table: Past and present . In: Journal of Chemical Education . 85, No. 4, 2008, pp. 585-589 (589). bibcode : 2008JChEd..85..585S . doi : 10.1021 / ed085p585 .
- ^ R. Alper: The simplified periodic table: elements ordered by their subshells . In: The Journal of Biological Physics and Chemistry . 10, No. 2, 2010, pp. 74-80. doi : 10.4024 / 43AL09F.jbpc.10.02 .
- ↑ E. Scerri : Some comments on the recently proposed periodic table featuring elements ordered by their subshells . In: Journal of Biological Physics and Chemistry . 12, No. 2, 2012, pp. 69-70.
- ^ HA Bent, F. Weinhold: Supporting information: News from the periodic table: An introduction to "Periodicity symbols, tables, and models for higher-order valency and donor-acceptor kinships" . In: Journal of Chemical Education . 84, No. 7, 2007, pp. 3-4. doi : 10.1021 / ed084p1145 .
- ↑ Eric Scerri: The periodic table: A very short introduction. Oxford University Press, New York 2011. ISBN 978-0-19-958249-5 .
- ^ M. Francl: Table manners Archived from the original on October 25, 2012. In: Nature Chemistry . 1, No. 2, May 2009, pp. 97-98. bibcode : 2009NatCh ... 1 ... 97F . doi : 10.1038 / nchem.183 . PMID 21378810 .
- ^ J. Emsley, R. Sharp: The periodic table: Top of the charts Archived from the original on July 1, 2017. In: The Independent . June 21, 2010.
- ^ G. Seaborg: Plutonium: The Ornery Element . In: Chemistry . 37, No. 6, 1964, p. 14.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the Elements. 2nd ed., Elsevier, Oxford 2016, ISBN 978-0-7506-3365-9 , p. 30: “In addition to the prediction of new elements and their probable properties, the periodic table has proved invaluable in suggesting fruitful lines of research in the preparation of new compounds. Indeed, this mode of thinking is now so ingrained in the minds of chemists that they rarely pause to reflect how extraordinarily difficult their task would be if periodic trends were unknown. It is the ability to anticipate the effect of changing an element or a group in a compound which enables work to be planned effectively, though the prudent chemist is always alert to the possibility of new effects or unsuspected factors which might surprisingly intervene. "
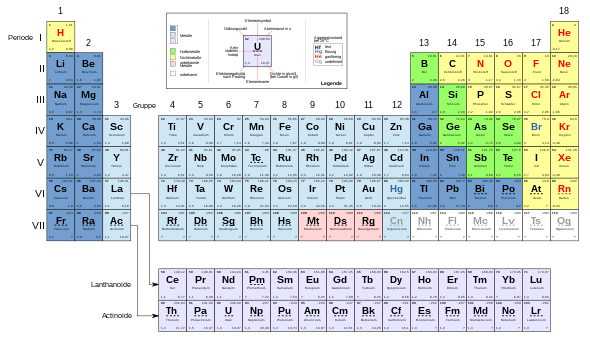

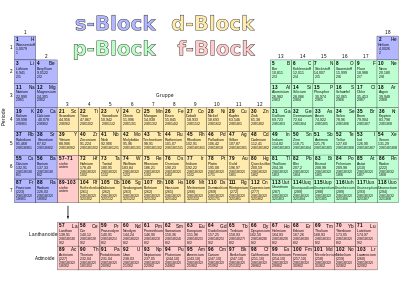



























![{\ displaystyle \ mathrm {{} _ {4} Be} \ quad {\ xrightarrow [{+ 8}] {}} \ quad \ mathrm {{} _ {12} Mg} \ quad {\ xrightarrow [{+ 8 }] {}} \ quad {\ color {BrickRed} \ mathbf {{} _ {20} Ca} \ quad {\ xrightarrow [{\ mathbf {+18}}] {}} \ quad \ mathbf {{} _ {38} Sr} \ quad {\ xrightarrow [{\ mathbf {+18}}] {}} \ quad \ mathbf {{} _ {56} Ba}} \ quad {\ xrightarrow [{+ 32}] {} } \ quad \ mathrm {{} _ {88} Ra}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b244a79c0b31c8833e988dd5f2b459e0547e8c22)