Alternative periodic tables
Alternative periodic tables differ from the traditional periodic table in the representation and assignment of the chemical elements . Since a systematic connection between atomic mass and chemical properties of the elements was discovered, there have been several alternative proposals to the order of the elements established by Dimitri Iwanowitsch Mendelejew (1834–1907) and Lothar Meyer (1830–1895). However, this periodic table is the commonly used periodic table in chemistry .
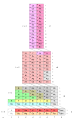
Janet periodic table
Charles Janet created a periodic table in which the elements were arranged in blocks. The chemical elements are grouped in a block according to the energetic orbitals of their electron shell. This periodic table is still sometimes used in physics today.
| f block | d block | p block | s block | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 H. |
2 He |
||||||||||||||||||||||||||||||
|
3 li |
4 Be |
||||||||||||||||||||||||||||||
|
5 B |
6 C |
7 N. |
8 O |
9 F. |
10 Ne |
11 Well |
12 mg |
||||||||||||||||||||||||
|
13 Al |
14 Si |
15 p |
16 pp |
17 cl |
18 ares |
19 K |
20 approx |
||||||||||||||||||||||||
|
21 Sc |
22 Ti |
23 V |
24 Cr |
25 mn |
26 feet |
27 Co |
28 Ni |
29 Cu |
30 notes |
31 Ga |
33 As |
35 Br |
36 kr |
37 Rb |
38 Sr |
||||||||||||||||
|
39 Y |
40 Zr |
41 Nb |
42 Mon |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 in |
50 Sn |
52 te |
53 I. |
54 Xe |
55 Cs |
56 Ba |
|||||||||||||||
|
57 La |
58 Ce |
59 Pr |
60 Nd |
61 pm |
62 Sm |
63 Eu |
64 Gd |
65 p |
66 Dy |
67 Ho |
68 he |
69 Tm |
70 yb |
71 Lu |
72 Hf |
73 days |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 ed |
81 Tl |
82 Pb |
83 bi |
86 para |
87 Fr |
88 Ra |
||
|
89 Ac |
90 th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 am |
96 cm |
97 Bk |
98 Cf |
99 it |
100 m |
101 Md |
102 No. |
103 Lr |
104 para |
105 Db |
106 Sg |
107 hours |
108 ms |
109 m |
110 Ds |
111 Rg |
112 cn |
113 Nh |
114 bottles |
115 Mc |
116 Lv |
117 Ts |
118 above |
||
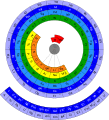
Elements spiral
Theodor Benfey developed a system in which the chemical elements are arranged in a spiral. Hydrogen is the center of the spiral. The elements with increasing mass are arranged around the center.
More similar concepts
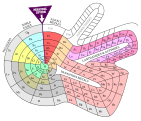
Flowers periodic table
The flower periodic table is spatial. The elements are shown as a wound tape in ascending order. The subgroup elements as well as the actinides and the lanthanoids each form a loop next to the main group elements .
Flower Periodic Table by Dmitri Mendeleev
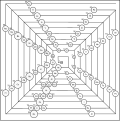
The Stowe Periodic Table
This periodic table is also three-dimensional. The periodic table has three axes which are the principal quantum number , the quantum number , and the magnetic quantum number represent. The periodic table is named after its inventor Timothy Stowe.
Bettermann's periodic table
The Bettermann periodic table is derived from the isoelectronic series of the elements. Eugenie Lisitzin showed in 1936 that this series can be represented as a polynomial. The valence electrons of the atoms also show a similar behavior.
Others
Web links
- Volker Mrasek : Representation of elements - hydrogen in alternative periodic tables. In: Deutschlandfunk broadcast “ Science in focus ”. 3rd March 2019 .
Individual evidence
- ↑ Hans-Jürgen Quadbeck-Seeger The world of the elements - The elements of the world. Wiley-VCH Verlag, 2006, ISBN 3-527-31789-9 , p. 107.
- ^ Albert Tarantola: Periodic Table of the Elements (Janet form). In: ipgp.fr . September 23, 2002, accessed January 2, 2013 .
- ↑ Periodic table with a difference. In: welsch.com. 2002, archived from the original on October 14, 2016 ; accessed on March 6, 2019 .
- ↑ The other periodic table. In: per-table.com. Archived from the original on November 18, 2013 ; Retrieved May 3, 2013 .