Heptanes
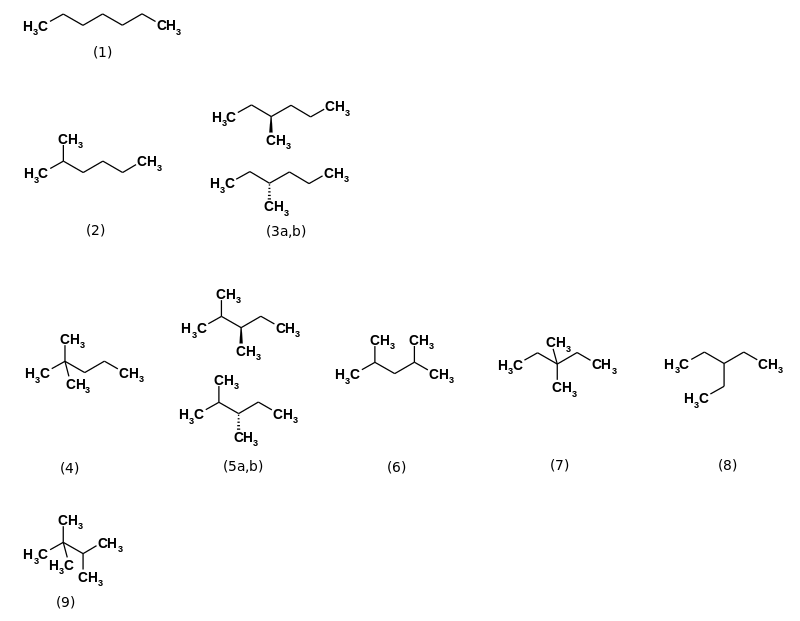
Heptane are among the alkanes counting hydrocarbons having the empirical formula C 7 H 16 . There are nine constitutional isomers :
- n -heptane
- 2-methylhexane
- 3-methylhexane
- 2,2-dimethylpentane
- 2,3-dimethylpentane
- 2,4-dimethylpentane
- 3,3-dimethylpentane
- 3-ethylpentane
- 2,2,3-trimethylbutane
3-methylhexane and 2,3-dimethylpentane are chiral compounds of which two enantiomers exist; The center of chirality is C3, which has hydrogen as a substituent and a methyl, ethyl and propyl or isopropyl radical. They are the simplest of the chiral alkanes.
| Comparison of physical properties of heptanes | |||||||||
| Surname | n -heptane | 2-methylhexane | 3-methylhexane | 2,2-dimethylpentane | 2,3-dimethylpentane | 2,4-dimethylpentane | 3,3-dimethylpentane | 3-ethylpentane | 2,2,3-trimethylbutane |
| other names | Triptan | ||||||||
| Structural formula | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8th) | (9) |
| CAS number | 142-82-5 | 591-76-4 | 589-34-4 | 590-35-2 | 565-59-3 | 108-08-7 | 562-49-2 | 617-78-7 | 464-06-2 |
| 78918-91-9 [( R ) -enantiomer ] | |||||||||
| 6131-24-4 [( S ) -enantiomer ] | |||||||||
| PubChem | 8900 | 11582 | 11507 | 11542 | 11260 | 7907 | 11229 | 12048 | 10044 |
| Molecular formula | C 7 H 16 | ||||||||
| Molar mass | 100.21 g mol −1 | ||||||||
| Brief description | colorless liquids | ||||||||
| Melting point | −91 ° C | −118 ° C | −119 ° C (racemate) | −123 ° C | −135 ° C | approx. −120 ° C | −135 ° C | −118.3 ° C | −25 ° C |
| boiling point | 98 ° C | 90 ° C | 92 ° C | 79 ° C | 90 ° C | 80 ° C | 86 ° C | 93 ° C | 81 ° C |
| Vapor pressure (20 ° C) | 47.4 hPa | 111 hPa | 71.8 hPa | 191 hPa (37.7 ° C) |
|||||
| Vapor pressure (30 ° C) | 78.1 hPa | 173 hPa | 115 hPa | ||||||
| Vapor pressure (50 ° C) | 189 hPa | 275 hPa | 255 hPa | 383 hPa | 264 hPa | 365 hPa | 235 hPa | 385 hPa | |
| density | 0.68 g cm −3 | 0.68 g cm −3 | 0.69 g cm −3 | 0.67 g cm −3 | 0.70 g cm −3 | 0.67 g cm −3 | 0.69 g cm −3 | 0.70 g cm −3 | 0.69 g cm −3 |
| solubility | 2.2 mg l −1 (25 ° C) | 2.5 mg l −1 (25 ° C) | 2.6 mg l −1 (25 ° C) | 4.4 mg l −1 (25 ° C) | 5.3 mg l −1 (25 ° C) | 4.4 mg l −1 (25 ° C) | 5.9 mg l −1 (25 ° C) | approx. 5.7 mg l −1 (25 ° C) | |
| Flash point | −7 ° C | −10 ° C | approx. −11 ° C | approx. −21 ° C | approx. −12 ° C | <−20 ° C | approx. −15 ° C | −18 ° C | approx. −19 ° C |
| Lower explosion limit (LEL) | 0.84% by volume | 1.0% by volume | 1% by volume | approx. 0.9% by volume | 1.1% by volume | approx. 0.9% by volume | 0.9% by volume | ||
| 35 g m −3 | 42 g m −3 | approx. 40 g m −3 | 45 g m −3 | approx. 40 g m −3 | 40 g m −3 | ||||
| Upper explosion limit (UEL) | 6.7% by volume | 6.0% by volume | 7% by volume | 6.9% by volume | 6.8% by volume | approx. 6.9% by volume | 6.9% by volume | ||
| 280 g m −3 | 250 g m −3 | 285 g m −3 | 280 g m −3 | approx. 285 g m −3 | 285 g m −3 | ||||
| Ignition temperature | 220 ° C | 280 ° C | 280 ° C | 320 ° C | 330 ° C | approx. 325 ° C | approx. 320 ° C | 450 ° C | |
Historical
Carl Schorlemmer was one of the first to investigate the isomers of heptane in more detail . In 1873 he described z. B. 3,3-dimethylpentane (called by him "dimethyl diethyl methane") and 3- ethyl pentane ("triethyl methane").
Individual evidence
- ↑ all data was taken from the GESTIS substance database , the individual links are in the name line.
- ↑ Entry on heptane in the GESTIS substance database of the IFA , accessed on July 26, 2019(JavaScript required) .
- ↑ Entry on 2-methylhexane in the GESTIS substance database of the IFA , accessed on July 26, 2019(JavaScript required) .
- ↑ Entry for CAS no. 589-34-4 in the GESTIS substance database of the IFA , accessed on July 26, 2019(JavaScript required) .
- ↑ Entry for CAS no. 590-35-2 in the GESTIS substance database of the IFA , accessed on July 28, 2019(JavaScript required) .
- ↑ Entry for CAS no. 565-59-3 in the GESTIS substance database of the IFA , accessed on July 28, 2019(JavaScript required) .
- ↑ Entry for CAS no. 108-08-7 in the GESTIS substance database of the IFA , accessed on July 29, 2019(JavaScript required) .
- ↑ Entry for CAS no. 562-49-2 in the GESTIS substance database of the IFA , accessed on July 29, 2019(JavaScript required) .
- ↑ Entry for CAS no. 617-78-7 in the GESTIS substance database of the IFA , accessed on July 29, 2019(JavaScript required) .
- ↑ Entry for CAS no. 464-06-2 in the GESTIS substance database of the IFA , accessed on July 29, 2019(JavaScript required) .
- ↑ Streiff, AJ; Murphy, ET; Sedlak, VA; Willingham, CB; Rossini, FD: Purification, Purity, and Freezing Points of 7 Heptanes, 16 Octanes, 6 Pentene, Cyclopentene, and 7 C9H12 Alkylbenzenes of the API-Standard and API-NBS Series in J. Res. Natl. Bur. Stand. (US) 37 (1946) 331.
- ↑ Carl Schorlemmer: About the heptanes in stone oil . In: F. Wöhler, J. Liebig, H. Kopp, E. Erlenmeyer, J. Volhard (Eds.): Justus Liebigs Annalen der Chemie . tape 166 , no. 2 . CF Winter'sche Verlagshandlung / Wiley-VCH, Leipzig and Heidelberg 1873, p. 172–178 , doi : 10.1002 / jlac.18731660207 ( online at the Bayerische Staatsbibliothek BSB / Munich Digitization Center (MDZ) [accessed on January 14, 2016]).