2,2,3-trimethylbutane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
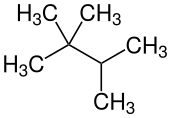
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,2,3-trimethylbutane | |||||||||||||||
| other names |
Triptan |
|||||||||||||||
| Molecular formula | C 7 H 16 | |||||||||||||||
| Brief description |
colorless, highly flammable liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.21 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.69 g cm −3 |
|||||||||||||||
| Melting point |
−25.45 ° C |
|||||||||||||||
| boiling point |
81 ° C |
|||||||||||||||
| Vapor pressure |
385 mbar (50 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (5.7 mg l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.3864 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2,2,3-Trimethylbutane is a chemical compound from the group of aliphatic saturated hydrocarbons . It is one of the nine constitutional isomers of heptane .
Extraction and presentation
2,2,3-Trimethylbutane occurs in petroleum . The compound can be produced from cracking gases or by hydrodesalkylation of isooctane and, in small proportions, by isomerization of n-heptane . As early as 1927 a laboratory synthesis was described in which the Grignard compound from tert-butyl chloride is reacted with acetone . The resulting 2,2,3-trimethylbutane-3-ol is the 2,3,3-Trimethylbut-1-en dehydrated and then by means of nickel catalyst for 2,2,3-trimethylbutane hydrogenated .
properties
Physical Properties
2,2,3-Trimethylbutane is a highly flammable and colorless liquid. The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.9222, B = 1203.362 and C = −46.776 in the temperature range from 286 to 355 K The temperature dependence of the enthalpy of vaporization can be calculated according to the equation Δ V H 0 = Aexp (−βT r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / Describe T c ) reduced temperature) with A = 46.76 kJ / mol, β = 0.2726 and T c = 531.1 K in the temperature range between 298 K and 353 K. Two polymorphic crystal forms can occur in the solid phase . Below −151.75 ° C there is crystal form II, above this temperature there is crystal form I. The enthalpy of transformation of the solid phase transition is 2.242 kJ · mol −1 . The crystal form I melts at −25.45 ° C.
The most important thermodynamic properties are listed in the following table:
| property | Type | Value [unit] |
|---|---|---|
| Standard enthalpy of formation | Δ f H 0 gas Δ f H 0 liquid |
−205.9 kJ mol −1 −238 kJ mol −1 |
| Enthalpy of combustion | Δ c H 0 liquid | −4803 kJ mol −1 |
| Heat capacity | c p | 213.51 J mol −1 K −1 (25 ° C) as a liquid |
| Enthalpy of fusion | Δ f H 0 | 2.2 kJ mol −1 at the melting point |
| Entropy of fusion | Δ f S 0 | 8.88 kJ mol −1 at the melting point |
| Enthalpy of evaporation | Δ V H 0 | 28.9 kJ mol −1 at the normal pressure boiling point of 32.19 kJ mol −1 at 25 ° C |
| Critical temperature | T C | 257.9 ° C |
| Critical pressure | P C | 29.5 bar |
| Critical volume | V C | 0.398 l mol −1 |
| Critical density | ρ C | 2.51 mol·l −1 |
Safety-related parameters
2,2,3-Trimethylbutane forms highly flammable vapor-air mixtures. The compound has a flash point of −19 ° C. The ignition temperature is 450 ° C. The substance therefore falls into temperature class T2.
use
2,2,3-Trimethylbutane is used as a high-quality, very knock- resistant aircraft fuel with an octane number of 112. The substance also serves as a reference substance in gas chromatography .
Individual evidence
- ↑ a b c d e f g h i j Entry on 2,2,3-trimethylbutane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d Domalski, ES; Hearing, ED: Heat Capacities and Entropies of Organic Compounds in the Condensed Phase. Volume III . In: J. Phys. Chem. Ref. Data 25 (1996) 1-525. doi : 10.1063 / 1.555985 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-506.
- ↑ Lindeman, LP; Annis, JL: Use of a conventional mass spectrometer as a detector for gas chromatography in Anal. Chem. 32 (1960) 1742-1747, doi : 10.1021 / ac50153a011 .
- ↑ a b c d Entry on 2,2,3-trimethylbutane. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ Haensel, V .; Donaldson, GR: Platforming of Pure Hydrocarbons in Ind. Eng. Chem. 43 (1951) 2102-2104, doi : 10.1021 / ie50501a036 .
- ↑ Blomsma, E .; Martens, YES; Jacobs, PA: Reaction Mechanisms of Isomerization and Cracking of Heptane on Pd / H-Beta Zeolite in J. Catal. 155 (1995) 141-147, doi : 10.1006 / jcat.1995.1195 .
- ↑ Edgar, G .; Calingaert, G .; Marker, RE: The preparation and properties of the isomeric heptanes. Part I. Preparation in J. Am. Chem. Soc. 51 (1929) 1483-1491, doi : 10.1021 / ja01380a027 .
- ↑ Forziati, AF; Norris, WR; Rossini, FD: Vapor Pressures and Boiling Points of Sixty API-NBS Hydrocarbons in J. Res. Natl. Bur. Stand. (US) 43 (1949) 555-567.
- ↑ a b c Majer, V .; Svoboda, V .: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation , Blackwell Scientific Publications, Oxford, 1985, p. 300.
- ↑ a b c Huffman, HM; Gross, ME; Scott, DW; McCullough, IP: Low temperature thermodynamic properties of six isomeric heptanes in J. Phys. Chem. 65 (1961) 495-503, doi : 10.1021 / j100821a026 .
- ↑ a b c Davies, GF; Gilbert, EC: Heats of combustion and formation of the nine isomeric heptanes in the liquid state in J. Am. Chem. Soc. 63 (1941) 2730-2732, doi : 10.1021 / ja01855a064 .
- ↑ a b c d Daubert, TE: Vapor-Liquid Critical Properties of Elements and Compounds. 5. Branched Alkanes and Cycloalkanes in J. Chem. Eng. Data 41 (1996) 365-372, doi : 10.1021 / je9501548 .




