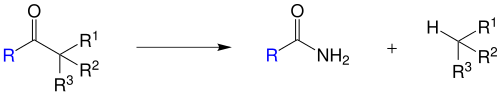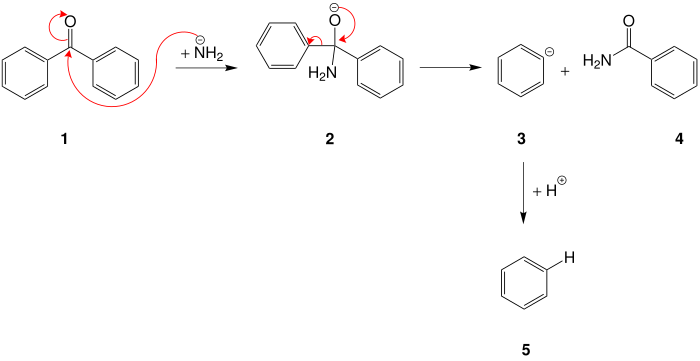Haller-Bauer reaction
The Haller-Bauer reaction is a name reaction in organic chemistry and was discovered in 1908 by chemists Albin Haller (1849–1925) and E. Bauer. It is a cleavage of non- enolizable ketones into alkane and carboxamide , which is why the reaction is also known as Haller-Bauer cleavage .
Overview reaction
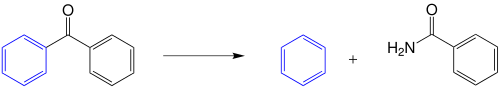
In 1908 Haller and Bauer discovered that benzophenone was split into benzamide and benzene by adding sodium amide NaNH 2 as a base and then water as an acid :
The two chemists carried out this observation in further experiments, so that the Haller-Bauer reaction could be generalized for non-enolizable ketones. The radicals R 1 , R 2 and R 3 can all be the same or different and consist, for example, of alkyl and / or aryl groups . The radical R is in most cases an aryl group. It is important to know how each residue affects the stability of the molecule in order to obtain the desired reaction products. If one of the radicals R 1 to R 3 is suitable for stabilizing a negative charge, aromatic carboxamide is preferably formed:
If the residues are unsuitable for stabilization, an aromatic hydrocarbon will be formed:
In the case of optically active starting materials, the reaction proceeds while maintaining the configuration.
mechanism
The mechanism has not been fully explored, but can be shown using the example of the cleavage of benzophenone to benzamide and benzene. The amide ion of the sodium amide attacks the carbonyl carbon atom of benzophenone 1 , so that a tetrahedral intermediate 2 is formed. In a further step is phenyl - anion 3 cleaved and there arises benzamide ( 4 ). The phenyl anion is neutralized by aqueous work-up and the second reaction product benzene ( 5 ) is formed.
Individual evidence
- ↑ a b M. B. Smith: March's Advanced Organic Chemistry - Reactions, Mechanisms, and Structure . 6th edition. John Wiley & Sons, 2013, ISBN 978-0-470-46259-1 , pp. 725 .
- ^ A. Haller, E. Bauer: Sur les produits de la réaction de l'amidure de sodium sur les cétones. In: Compt. Rend. tape 147 , 1908, pp. 824-826 .
- ↑ JP Gilday, LA Paquette: Carbon-Carbon Bond Cleavage by the Haller-Bauer and Related Reactions. A review . In: Organic Preparations and Procedures International: The New Journal for Organic Synthesis . tape 22 , no. 2 , 1990, p. 167-201 .
- ↑ W. Kunz, H. Krauch: Reactions of organic chemistry . Wiley-VCH, 1997, ISBN 978-3-527-29713-9 , pp. 81-82 .
- ^ Jie Jack Li: Name Reactions - A Collection of Detailed Reaction Mechanism . Springer, 2006, ISBN 978-3-540-30030-4 , pp. 279-280 .