Nitro compounds
Nitro compounds are organic compounds in which one or more nitro groups (-NO 2 ) are bonded to one carbon atom of an organic radical. A distinction can be made between aliphatic and aromatic nitro compounds. The aromatic nitro compounds are usually more stable. Organic compounds in which the nitro group is bound to another nitrogen atom (so-called N- nitro compounds) belong to a separate class of compounds and are called nitramines .
Aromatic nitro compounds
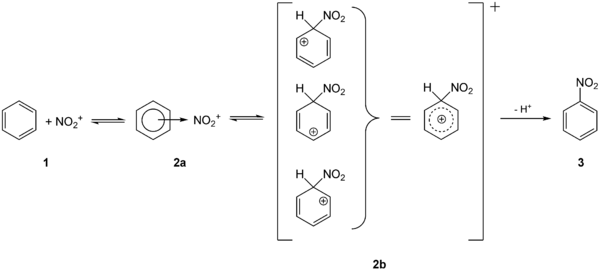
The aromatic nitro compounds (often also nitroaromatics ) can be produced directly by electrophilic aromatic substitution with nitrating acid . The nitration of benzene provides the simplest nitroaromatic, nitrobenzene 3 , via the intermediates 2a and 2b :
The best known representatives include the explosives trinitrotoluene , picric and styphnic acid . In addition to explosives, the group of nitroaromatics also includes dyes , pharmaceuticals , herbicides , raw materials for the plastics industry and synthetic fragrances (e.g. nitro musk fragrances, which have now fallen into disrepute). In the chemical industry, nitroaromatics are important intermediate products, as they are required for the production of aromatic amines (e.g. aniline , which is synthesized from nitrobenzene) - see also BASF .
Nitro aromatics as environmental toxins
Many nitroaromatics are environmental toxins and as such represent a ( waste ) problem. In Germany, for example, the soil and groundwater in the vicinity of former explosives factories are still contaminated with aromatic nitro compounds.
Aliphatic nitro compounds
Aliphatic nitro compounds can u. a. by nucleophilic substitution of alkyl halides with inorganic nitrites , e.g. B. silver nitrite , AgNO 2 or sodium nitrite , NaNO 2 produce.

- Synthesis of nitromethane from chloroacetic acid and sodium nitrite.
They can be used in many different ways. For example, they can be used in a Henry reaction (nitro- aldol reaction ). The nitro compounds obtained in this way can also be converted in further reaction steps by reduction to aliphatic amines or in a Nef reaction to give ketones . They are important as intermediate products in the chemical industry, as solvents or fuel .
Differentiation between nitro and nitrite groups
In the production of nitro compounds, the ester of nitrous acid ( nitrites ) is always a by-product in a side reaction . The percentage, however, depends on the reaction route. The difference lies in the value of nitrogen. The nitro group is bound to the carbon atom via the nitrogen atom . In the isomeric nitrite group, nitrogen is trivalent and bonded to carbon via an oxygen atom. Nitroalkanes are temperature stable than Nitritester.
Individual evidence
- ↑ Bavarian State Office for Environmental Protection: Legacy Armaments - Technical information and exchange of experience (PDF; 3.0 MB), specialist conference on October 14, 2004.