Larock indole synthesis
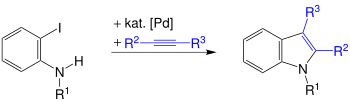
The Larock indole synthesis is a name reaction of organic chemistry , which was discovered in 1991 by the American professor of organic chemistry Richard C. Larock . The reaction is used for the synthesis of indoles by palladium-catalyzed annulation from o -iodine anilines and disubstituted alkynes .
Overview reaction
The Larock indole synthesis is a one-pot reaction that produces 2,3-disubstituted indoles. For this purpose, Larock used o -iodaniline and its derivatives with alkyl, aryl or hydrogen radicals R 1 and disubstituted alkynes, the radicals R 2 and R 3 of which can be alkyl , aryl , alkenyl , alkoxy or silyl groups . The best results can be achieved by using carbonates or acetates as the base and by adding lithium chloride .
This indole synthesis proceeds regioselectively and is influenced by the steric demands of the alkyne residues. As a result, the sterically more demanding residue is always in the ortho position of the indole formed after the reaction .
mechanism
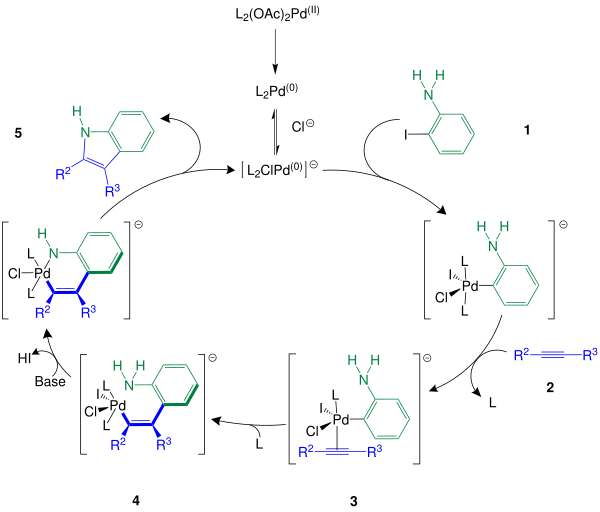
The mechanism begins with the reduction of the palladium (II) catalyst ( L 2 (OAc) 2 Pd ) to the palladium (0) complex and is similar to the mechanism of the Heck reaction . As ligand L can, for example, triphenylphosphine may be used. After the palladium (0) complex has been stabilized with the aid of the chloride ion , the catalytic cycle can begin with the oxidative addition of the o -iodoaniline 1 to the stabilized palladium (0) complex. A regioselective syn - insertion of the palladium into the carbon-iodine bond occurs, so that a new palladium complex is formed. This next reacts with the alkyne 2 , which leads to the formation of the π- alkyne- σ -organopalladium complex 3 between the alkyne and the palladium species. It is 3 aligned such that the more sterically demanding rest of the alkyne not for direct neighbors of the organic group is. At the same time as the addition step, a ligand emerges from the complex compound . This is followed by a carbopalladation of the C≡C triple bond and the ligand that has escaped is taken up again into the complex. The carbopalladation intermediate 4 is formed . In the next step, the nitrogen atom of the amino group attacks the palladium atom in a nucleophilic manner , while at the same time hydrogen iodide is split off from complex 4 by the addition of the base - such as potassium carbonate (K 2 CO 3 ) . A six-membered ring is formed. At the end of the catalytic cycle, reductive elimination takes place , which leads to the formation of the indole 5 and a restoration of the palladium (0) complex.
The organyl radicals R 2 and R 3 correspond to the radicals mentioned in the overview reaction section .
Individual evidence
- ↑ RC Larock, EK Yum: Synthesis of Indoles via Palladium-Catalyzed Heteroannulation of Internal Alkynes . In: J. Am. Chem. Soc . tape 113 , 1991, pp. 6689-6690 , doi : 10.1021 / ja00017a059 .
- ↑ GW Gribble: Recent developments in indole ring synthesis - methodology and applications . In: J. Chem. Soc., Perkin Trans. 1 . tape 1 , 2000, pp. 1045-1075 , doi : 10.1039 / a909834h .
- ↑ L. Kürti, B. Czakó: Strategic Applications of Named Reactions in Organic Synthesis - Background and Detailed Mechanisms . Elsevier Inc., 2005, ISBN 978-0-12-369483-6 , pp. 260-261 .
- ↑ RC Larock, EK Yum: Synthesis of Indoles via Palladium-Catalyzed Heteroannulation of Internal Alkynes . In: J. Am. Chem. Soc . tape 113 , 1991, pp. 6689-6690 , doi : 10.1021 / ja00017a059 .
- ↑ RC Larock, EK Yum, MD Refvik: Synthesis of 2,3-Disubstituted Indoles via Palladium-Catalyzed Annulation of Internal Alkynes . In: J. Org. Chem . tape 63 , 1998, pp. 7652-7662 , doi : 10.1021 / jo9803277 .
- ↑ L. Kürti, B. Czakó: Strategic Applications of Named Reactions in Organic Synthesis - Background and Detailed Mechanisms . Elsevier Inc., 2005, ISBN 978-0-12-369483-6 , pp. 260-261 .