Silyl protecting group
The term silyl protective group covers a whole range of chemical compounds that can be used to convert a reactive functional group into an unreactive form using an organosilicon compound and thus to protect it from an undesired reaction. As a rule, silyl radicals are used as a protective group for the hydroxyl group in alcohols or phenols .
use
Hydroxy groups in alcohols are nucleophilic functional groups and are therefore attacked by electrophiles or removed in an elimination reaction . To prevent this in undesired cases, the protective group technique was introduced in organic chemistry. A silyl protective group turns the alcohol with the hydroxyl group into a relatively stable and unreactive derivative of the alcohol, a silyl ether in which the proton of the hydroxyl group is replaced by a substituted silicon radical.
Hydroxy groups protected by a silyl group can be converted directly into aldehydes , ketones , bromides , acetates and ethers without a previous deprotection step.
presentation
Silyl ethers are usually produced by reacting the corresponding silyl chlorides or silyl sulfates with alcohols or phenols in the presence of bases. Little nucleophilic bases such as triethylamine or diisopropylethylamine (Hünig base) are used as the base. Usually, dry and aprotic organic solvents such as dichloromethane are used . There have been extensive studies of the stability of the silyl ethers. They are also used to a considerable extent.
The simplest and cheapest silane known as a reagent for introducing the silyl protective group is trimethylchlorosilane (middle in the above figure, R 2 = R 3 = R 4 = CH 3 ). It is a by-product of the Rochow and Müller silicone production. The higher silyl halides are specifically synthesized for use as a protective group. In addition to the trimethylsilyl group, important representatives are the more highly substituted silanes such as. B. tert -Butyldimethylsilyl and triethylsilyl groups.
stability
With increasing steric hindrance on the silicon atom, the stability of the protective group also increases. The trimethylsilyl group is often very labile and is already partially split by silica gel during chromatography. However, the higher silyl ethers are stable to chromatographic conditions. Silyl protective groups can be cleaved by acids or better and more selectively by fluoride ions. Potassium fluoride , tetrabutylammonium fluoride (TBAF) or hydrofluoric acid can serve as the fluoride source .
Silyl groups are the only protecting groups that are cleaved by fluoride ions. This unique property means that silyl protective groups can be cleaved very selectively alongside all other possible protective groups.
| Surname | formula | abbreviation | cleavage |
|---|---|---|---|
| Trimethylsilyl | 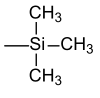 |
TMS | Potassium fluoride , acetic acid or potassium carbonate in methanol |
| Triethylsilyl |  |
TES | 10 to 100 times more stable than a TMS group; Trifluoroacetic acid in water / tetrahydrofuran , acetic acid in water / tetrahydrofuran, hydrofluoric acid , pyridinium hydrofluoride in pyridine |
| tert -butyldimethylsilyl |  |
TBS, TBDMS | Acetic acid in tetrahydrofuran / water, pyridinium tosylate in methanol, trifluoroacetic acid in water, hydrofluoric acid in acetonitrile , pyridinium hydrofluoride in tetrahydrofuran, tetrabutylammonium fluoride in THF |
| Triisopropylsilyl |  |
TIPS | Under the same conditions as TBS but longer reaction times; Tetrabutylammonium fluoride in tetrahydrofuran, hydrofluoric acid in acetonitrile, pyridinium hydrofluoride in tetrahydrofuran. |
| tert. -Butyldiphenylsilyl |  |
TBDPS | Under the same conditions as TBS but longer reaction times (100 to 250 times slower than TBS and 5 to 10 times slower than TIPS); Tetrabutylammonium fluoride in tetrahydrofuran, hydrofluoric acid in acetonitrile, pyridinium hydrofluoride in tetrahydrofuran |
literature
- Philip J. Kocieński: Protecting Groups , 1st edition, Georg Thieme Verlag, Stuttgart 1994, ISBN 3-13-135601-4 .
- Peter GM Wuts, Theodora W. Greene: Greene's Protective Groups in Organic Synthesis , 4th Edition, John Wiley & Sons Inc., Hoboken, New Jersey, ISBN 0-471-69754-0 .
- Entry on silyl protecting groups. In: Römpp Online . Georg Thieme Verlag, accessed on April 29, 2014.
- Michael Schelhaas, Herbert Waldmann: “Protection group strategies in organic synthesis”, in: Angewandte Chemie , 1996 , 103 , pp. 2192–2219; doi : 10.1002 / anie.19961081805 .
- Krzysztof Jarowicki, Philip Kocienski : “Protecting groups” , in: J. Chem. Soc., Perkin Trans. 1 , 1998 , pp. 4005-4037; doi : 10.1039 / A803688H .
Individual evidence
- ↑ GA Tolstikov, MS Miftakhov, NS Vostrikov, NG Komissarova, ME Adler, O. Kuznetsov: Zh. Org. Khim. 1988 , 24 , 224-225.
- ↑ FP Cossio, JM Aizpurua, C. Palomo: Synthetic applications of chromium (VI) reagents in combination with chlorotrimethylsilane . In: Canadian Journal of Chemistry . 64 (2), 1986, pp. 225-231, doi : 10.1139 / v86-039 .
- ↑ S. Kim, JH Park: J. Org. Chem. 1988 , 53 , 3111-3113.
- ↑ JM Aizpurua, FP Cossio, C. Palomo: J. Org. Chem. 1986 , 51 , 4941-4943.
- ^ SJ Danishefsky, NJ Mantlo: J. Am. Chem. Soc. 1988 , 110 , 8129-8133.
- ^ DG Saunders: Synthesis , 1988 , 377-378.
- ↑ PJ Kocienski: Protecting Groups , S. 29th
- ↑ PJ Kocienski: Protecting Groups , S. 31st
- ↑ Tod K Jones, Robert A. Reamer, Richard Desmond, Sander G. Mills: "Chemistry of tricarbonyl hemiketals and application of Evans technology to the total synthesis of the immunosuppressant (-) - FK-506", in: J. Am. Chem. Soc. , 1990 , 112 , pp. 2998-3017; doi : 10.1021 / ja00164a023 .
- ↑ Dieter Seebach, Hak-Fun Chow, Richard FW Jackson, Marius A. Sutter, Suvit Thaisrivongs, Jürg Zimmermann: "(+) - 11,11′-Di-O-methylelaiophylidene - preparation from elaiophylin and total synthesis from ( R ) -3-hydroxybutyrate and ( S ) -malate ”, in: Liebigs Ann. Chem. , 1986 , pp. 1281-1308; doi : 10.1002 / jlac.198619860714 .
- ↑ David A. Evans, Stephen W. Kaldor, Todd K. Jones, Jon Clardy, Thomas J. Stout: "Total synthesis of the macrolide antibiotic cytovaricin", in: J. Am. Chem. Soc. , 1990 , 112 , pp. 7001-7031; doi : 10.1021 / ja00175a038 .
- ↑ James A. Marshall, Richard Sedrani: “A convergent, highly stereoselective synthesis of a C-11-C-21 subunit of the macbecins”, in: J. Org. Chem. , 1991 , 56 , pp. 5496-5498; doi : 10.1021 / jo00019a004 .
- ↑ James D. White, Motoji Kawasaki: "Total synthesis of (+) - Latrunculin A", in: J. Am. Chem. Soc. , 1990 , 112 , pp. 4991-4993; doi : 10.1021 / ja00168a071 .
- ↑ Morris J. Robins, Vicente Samano, Mark D. Johnson: “Nucleic acid-related compounds. 58. Periodinane oxidation, selective primary deprotection, and remarkably stereoselective reduction of tert-butyldimethylsilyl-protected ribonucleosides. Synthesis of 9- (β-D-xylofuranosyl) adenine or 3'-deuterioadenosine from adenosine ”, in: J. Org. Chem. , 1990 , 55 , pp 410-412; doi : 10.1021 / jo00289a004 .
- ^ R. Roger F. Newton, Derek P. Reynolds, Colin F. Webb, Stanley M. Roberts: "A short and efficient total synthesis of (±) prostaglandin D 2 methyl ester involving a new method for the cleavage of a dimethyl- t-butylsilyl ether ”, in: J. Chem. Soc., Perkin Trans. 1 , 1981 , pp. 2055-2058; doi : 10.1039 / P19810002055 .
- ↑ Kyriacos C. Nicolaou, RA Daines, TK Chakraborty: "Total synthesis of amphoteronolide B", in: J. Am. Chem. Soc. , 1987 , 109 , pp. 2208-2210; doi : 10.1021 / ja00241a063 .
- ↑ Leo A. Paquette, Annette M. Doherty, Christopher M. Rayner: "Total synthesis of furanocembranolides. 1. Stereocontrolled preparation of key heterocyclic building blocks and assembly of a complete seco-pseudopterane framework “, in: J. Am. Chem. Soc. , 1991 , 109 , pp. 3910-3926; doi : 10.1021 / ja00036a045 .
- ↑ PJ Kocienski: Protecting Groups , p. 40
- ↑ PJ Kocieński: Protecting Groups , pp. 38-39.


