Disubstituted benzenes
| Disubstituted benzenes | ||||
| Surname | ortho | meta | para | Mixture of isomers |
| same substituents |

|

|

|

|
| various substituents |

|

|

|

|
The disubstituted benzenes form a large group of aromatic compounds . The structure consists of a benzene ring with two attached substituents , which can be either the same or different. Their different arrangement results in three constitutional isomers with the same empirical formula. Depending on the relative arrangement, a distinction is made between ortho , meta and para isomers.
Naming
Trunk connections
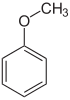
In most cases, the monosubstituted benzenes form new parent compound names : with –OH the phenol , with –NH 2 the aniline , with –OCH 3 the anisole , with –CH 3 the toluene . With the nitro group or halogen substituents, however, no new parent compound names are created.
Historical naming
If one looks at the combinations of substituents on aromatics using the example of the methyl group (–CH 3 ) and the hydroxyl group (–OH), one can see very different name combinations and forms of origin. In all cases the connections have common names.
- The three dimethylbenzenes have a common tribe name, namely xylene . The distinction is made only by specifying the substituent position, e.g. B. by marking with ortho- , meta- or para- .
- The three dihydroxybenzenes , however, do not have a common root name. The three isomers have three completely separate and different names - catechol , resorcinol and hydroquinone - which refer to their origin and discovery.
- In the third case of the combination of both substituents, the compounds can be viewed as methylphenols or hydroxytoluenes. In this case, these compounds have a new common name - cresol - which in turn goes back to their origin and discovery. The distinction is made e.g. B. again by marking with ortho- , meta- or para- .
|
Benzene (-H) |
Toluene (-CH 3 ) |
Phenol (-OH) |
|
Toluene (-CH 3 ) |
Dimethylbenzenes xylenes 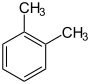  |
Methylphenols, hydroxytoluenes, cresols 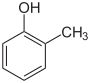  |
|
Phenol (-OH) |
Dihydroxybenzenes o : catechol , m : resorcinol , p : hydroquinone 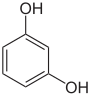 |
Systematic naming
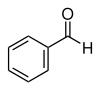
With a more systematic naming, the following ranking of the parent compounds can be determined: benzoic acid, benzaldehyde, benzyl alcohol, phenol, aniline, anisole, toluene, nitrobenzene, halobenzenes. When two master connections with their own name come together, z. B. from benzaldehyde and anisole the group of substances methoxybenzaldehydes, the para -isomer also has the common name anisaldehyde.
However, if nitro groups or halogen substituents are involved, the name of the parent compounds does not change. From toluene z. B. the nitrotoluenes, from benzaldehyde the bromobenzaldehydes.
The table contains a compilation of all combinations of very common substituents:
More connections
- Ethylmethylbenzenes - Diethylbenzenes - Divinylbenzenes - Xylyl bromides - Methylstyrenes
- Phenetidines - ethyl phenols - n -Propylphenole - Isopropylbenzaldehyde
- Sulfobenzoic acids
- Benzene dicarboxylic acids ( o : phthalic acid , m : isophthalic acid , p : terephthalic acid )
- Benzoldicarbaldehyde ( o : phthalaldehyde , m : isophthalaldehyde , p : terephthalaldehyde )
- Benzene dicarbonitrile ( o : phthalonitrile , m : isophthalonitrile , p : terephthalodinitrile )
- Nitrobenzoylchloride - Nitrobenzonitrile - Nitrobenzylchloride
- Chlorobenzotrifluoride - Chlorobenzotrichloride
presentation
The presentation succeeds by introducing z. B. a nitro group or halogen group, or by converting an already existing second substituent, e.g. B. by methylation of a phenol group to the methoxy group .
properties
Melting and boiling points
The boiling points of the three isomers are often close to one another, while their melting points differ significantly. The para isomer, which has the highest symmetry, usually has the highest melting point.
Acidity and basicity
The acidity and basicity of the parent compounds such as phenol, aniline and benzoic acid are influenced by their secondary substituents and their position. 2- and 4-nitrophenol have a lower pK compared to the 3-nitrophenol s value; their acidities are therefore greater. In the ortho and para form, the phenolate ion can shift a double bond to the electron-withdrawing nitro group ( −M effect ). The second O can form a negative center of gravity there. This is not possible with the meta form. The nitroanilines have (4.603) significantly lower pK opposite the aniline s values; the electron-withdrawing nitro group (−M effect) reduces the basicity. A proton can therefore be absorbed much more poorly.
Intramolecular hydrogen bonds
If intra- molecular hydrogen bridges can form, this has u. a. Effects on melting points and solubility in water.
intramolecular hydrogen bond 






2-nitrophenol 2-nitroaniline 2-nitrobenzaldehyde 2-aminobenzaldehyde Anthranilic acid Salicylaldehyde Salicylic acid
2-Nitrophenol and 2-Nitroaniline have the lowest melting point because they can form an intramolecular hydrogen bond. In contrast, the other two isomers form intermolecular hydrogen bonds. With 2-nitrophenol or 2-nitroaniline, energy is not required to break these bridges.
The nitrophenols are sparingly soluble in water, but the values differ within this group. The significantly poorer solubility of 2-nitrophenol in water can also be explained well by the intramolecular hydrogen bond. As a result, the molecule is significantly less polar towards the outside. In contrast, the solubilities of 3- and 4-nitrophenol are about the same and, in comparison, significantly better. Here now form rather between the phenolic hydroxyl and water inter -molecular hydrogen bonds.
See also
Individual evidence
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .