Nitrobenzaldehydes
| Nitrobenzaldehydes | ||||||
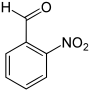
| Surname | 2-nitrobenzaldehyde | 3-nitrobenzaldehyde | 4-nitrobenzaldehyde | |||
| other names | o -nitrobenzaldehyde | m -nitrobenzaldehyde | p -nitrobenzaldehyde | |||
| Structural formula |
|

|

|
|||
| CAS number | 552-89-6 | 99-61-6 | 555-16-8 | |||
| PubChem | 11101 | 7449 | 541 | |||
| Molecular formula | C 7 H 5 NO 3 | |||||
| Molar mass | 151.12 g mol −1 | |||||
| Physical state | firmly | |||||
| Brief description | slightly yellow crystalline powder | |||||
| Melting point | 41-43 ° C | 55 ° C | 103-106 ° C | |||
| boiling point | 156 ° C (20 hPa) | 164 ° C (31 hPa) | - | |||
|
GHS labeling |
|
|
|
|||
| H and P phrases | 302-315-319-335 | 302-411 | 317-319-412 | |||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||
| 261-305 + 351 + 338 | 273 |
261-280-302 + 352 305 + 351 + 338 |
||||
In chemistry, nitrobenzaldehydes form a group of substances that are derived from both benzaldehyde and nitrobenzene . The structure consists of a benzene ring with attached aldehyde (-CHO) and nitro group (-NO 2 ). Their different arrangement ( ortho , meta or para ) results in three constitutional isomers with the empirical formula C 7 H 5 NO 3 .
presentation
- 2-Nitrotoluene is chlorinated when hot . The 2-nitrobenzylidene dichloride formed is saponified , with 2-nitrobenzaldehyde being formed.
- 3-Nitrobenzaldehyde is obtained from benzaldehyde by reaction with fuming nitric acid in the presence of concentrated sulfuric acid .
- 4-Nitrobenzaldehyde is obtained from 4-nitrotoluene by oxidation with chromium (VI) oxide in acetic anhydride . The resulting 4-nitrobenzaldiacetate is hydrolyzed with sulfuric acid in aqueous ethanol .
properties
The nitrobenzaldehydes are slightly yellow crystalline solids, some of which have a bitter almond odor. The 4-nitrobenzaldehyde, which has the highest symmetry, has the highest melting point.
In a basic medium, the nitrobenzaldehydes disproportionate in a Cannizzaro reaction to form nitrobenzoic acids and nitrobenzyl alcohols .
use
- 2-Nitrobenzaldehyde is used as a reagent on isopropanol and acetone .
- 2-Nitrobenzaldehyde reacts with acetone to form indigo .
Individual evidence
- ↑ a b c Entry on 2-nitrobenzaldehyde in the GESTIS substance database of the IFA , accessed on March 29, 2016(JavaScript required) .
- ↑ a b c Entry on 3-nitrobenzaldehyde in the GESTIS substance database of the IFA , accessed on March 29, 2016(JavaScript required) .
- ↑ a b c Entry on 4-nitrobenzaldehyde in the GESTIS substance database of the IFA , accessed on March 29, 2016(JavaScript required) .
- ↑ Test specification : Nitration of benzaldehyde to 3-nitrobenzaldehyde (PDF) from the collection of integrated organic-chemical internship at the University of Regensburg, accessed on October 30, 2011.
- ↑ University of Hamburg: 3-nitrobenzaldehyde, test instructions (PDF; 24 kB).
- ^ SV Lieberman and Ralph Connor: p-Nitrobenzaldehyde In: Organic Syntheses . 18, 1938, p. 61, doi : 10.15227 / orgsyn.018.0061 ; Coll. Vol. 2, 1943, p. 441 ( PDF ).
- ↑ Helmut Schmidt: "Indigo - 100 Years of Industrial Synthesis", in: Chemie in our Time , 1997 , 31 (3), pp. 121–128; doi: 10.1002 / ciuz.19970310304 .




