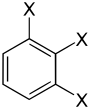
Trisubstituted benzenes
The trisubstituted benzenes form a large group of aromatic compounds . The structure consists of a benzene ring with three attached substituents , which can be either the same or different. Their different arrangement results in three substitution patterns with the same empirical formula . Depending on the arrangement, one speaks of vic. -, asym. or sym. isomer.
With the same substituents three constitutional isomers are formed , with two different six and with three different ten.
Naming
If one looks at the combinations of substituents on aromatics using the example of the methyl group (–CH 3 ) and the hydroxyl group (–OH), one can see very different name combinations and forms of origin. In all cases the connections have common names.
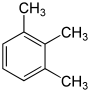
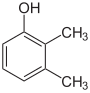
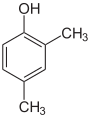
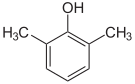
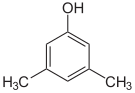
- The three trimethylbenzenes have no common tribe name. The three isomers have three completely separate and different names - hemellitol , pseudocumene and mesitylene - which refer to their origin and discovery.
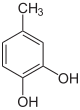
- The three trihydroxybenzenes also have no common tribe name. The three isomers also have three completely separate and different names - pyrogallol , hydroxyhydroquinone and phloroglucine - which also refer to their origin and discovery.
- In the third case of combining both substituents, there are two groups of compounds, each with six constitutional isomers:
- with two methyl groups and one hydroxyl group: They can be called dimethylphenols or hydroxylenes . This is where the common trivial name Xylenole developed.
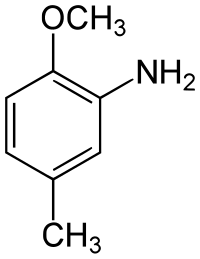
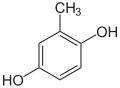
- with two hydroxyl groups and one methyl group: They can be understood as methyl derivatives of catechol (2 isomers), resorcinol (3 isomers) and hydroquinone (1 isomer). A uniform naming in this way is difficult because the dihydroxybenzenes do not form a common common name. They are therefore referred to as dihydroxytoluenes , since only -toluene forms a common new common tribe name . There is no common trivial name like the xylenols. Individual fabrics have individual names, such as B. Orcin (3,5-dihydroxytoluene).
|
Benzene (-H) |
2 × (-CH 3 ) | 2 × (-OH) | ||||||||||||
|
Toluene (-CH 3 ) |
Trimethylbenzenes
|
Dihydroxytoluenes
|
||||||||||||
|
Phenol (-OH) |
(Dimethylphenols / Hydroxyxylole) xylenols
|
Trihydroxybenzenes
|
Same substituents
| Trisubstituted benzenes | |||
| Surname | vic. | asym. | sym. |
| same substituents |
|

|

|
- Trimethylbenzenes
- Trihydroxybenzenes
- Trimethoxybenzenes
- Triaminobenzenes
- Trinitrobenzenes
- Trifluorobenzenes
- Trichlorobenzenes
- Tribromobenzenes
- Triiodobenzenes
- Benzenetricarboxylic acids
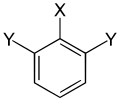
Two different substituents
| Trisubstituted benzenes | ||||||
| Surname | vic. | asym. | asym. | vic. | asym. | sym. |
| two different substituents |

|

|

|
|

|

|
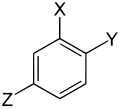
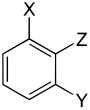
Three different substituents
| Trisubstituted benzenes | ||||||||||
| Surname | vic. | asym. | asym. | vic. | vic. | asym. | sym. | asym. | asym. | asym. |
| three different substituents |

|

|
|

|
|

|
|

|

|
|
- Hydroxymethoxybenzyl alcohols: vanillyl alcohol , isovanillyl alcohol , ortho-vanillyl alcohol , ...
- Hydroxymethoxybenzaldehyde: vanillin , isovanillin , ortho-vanillin , ...
- Hydroxymethoxybenzoic acids: vanillic acid , isovanillic acid , ortho-vanillic acid , ...
- Cresidines
- Nitrotoluidines
- Nitroanisidines
- Chlorocresols