Dichloroaniline
The dichloroanilines form a group of aromatic compounds that are derived from both aniline and chlorobenzene . The structure consists of a benzene ring with an attached amino group (–NH 2 ) and two chlorine (–Cl) as substituents . Their different arrangement results in six constitutional isomers with the empirical formula C 6 H 5 Cl 2 N. All substances are sparingly soluble in water and are toxic.
| Dichloroaniline | |||||||||||||||
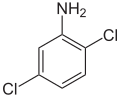
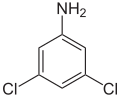
| Surname | 2,3-dichloroaniline | 2,4-dichloroaniline | 2,5-dichloroaniline | 2,6-dichloroaniline | 3,4-dichloroaniline | 3,5-dichloroaniline | |||||||||
| other names | 1-amino- 2,3-dichlorobenzene, 2,3-dichlorophenylamine, 2,3-dichlorobenzene amine, 2,3-DCA |
1-amino- 2,4-dichlorobenzene, 2,4-dichlorophenylamine, 2,4-dichlorobenzene amine, 2,4-DCA |
1-Amino- 2,5-dichlorobenzene, 2,5-dichlorophenylamine, 2,5-dichlorobenzene amine , 2,5-DCA, scarlet GG base |
1-amino- 2,6-dichlorobenzene, 2,6-dichlorophenylamine, 2,6-dichlorobenzene amine, 2,6-DCA |
1-amino- 3,4-dichlorobenzene, 3,4-dichlorophenylamine, 3,4-dichlorobenzene amine, 3,4-DCA |
1-amino- 3,5-dichlorobenzene, 3,5-dichlorophenylamine, 3,5-dichlorobenzene amine, 3,5-DCA |
|||||||||
| Structural formula |

|

|
|

|

|
|
|||||||||
| CAS number | 608-27-5 | 554-00-7 | 95-82-9 | 608-31-1 | 95-76-1 | 626-43-7 | |||||||||
| PubChem | 11844 | 11123 | 7262 | 11846 | 7257 | 12281 | |||||||||
| ECHA InfoCard | 100.009.235 | 100.008.235 | 100.002.233 | 100.009.237 | 100.002.227 | 100.009.954 | |||||||||
| Molecular formula | C 6 H 5 Cl 2 N | ||||||||||||||
| Molar mass | 162.02 g mol −1 | ||||||||||||||
| Physical state | firmly | ||||||||||||||
| Brief description | flammable, crystalline solids | ||||||||||||||
| Melting point | 23-24 ° C | 62 ° C | 50 ° C | 36-38 ° C | 70-72 ° C | 51-53 ° C | |||||||||
| boiling point | 252 ° C | 242 ° C | 251 ° C | 228 ° C | 272 ° C | 259-260 ° C | |||||||||
| density | 1.38 g cm −3 (at 20 ° C) | 1.56 g cm −3 (at 20 ° C) | 1.54 g cm −3 (at 20 ° C) | 1.28 g cm −3 (at 20 ° C) | 1.57 g cm −3 (at 20 ° C) | 1.58 g cm −3 (at 20 ° C) | |||||||||
| solubility | 0.75–1 g · l −1 (at 20 ° C) | 0.45 g l −1 (at 20 ° C) | 0.56 g l −1 (at 20 ° C) | 1.6 g l −1 (at 20 ° C) | 0.58 g l −1 (at 20 ° C) | 0.78 g l −1 (at 20 ° C) | |||||||||
| Flash point | 115 ° C | 115 ° C | 139 ° C | 118 ° C | 135 ° C | 133 ° C | |||||||||
| Ignition temperature | > 600 ° C | > 540 ° C | > 600 ° C | > 600 ° C | 620 ° C | ||||||||||
|
GHS labeling |
|
|
|
||||||||||||
| H and P phrases | 301-311-331-373-410 | 301-311-331-317-318-410 | 301-311-331-373-410 | ||||||||||||
| no EUH phrases | |||||||||||||||
|
261-273-280- 301 + 310-501 |
260-273-280-301 + 310 + 330- 361 + 364-403 + 233 |
261-273-280- 301 + 310-311-501 |
261-273-280- 301 + 310-311-501 |
261-273-280-301 + 310- 305 + 351 + 338-311 |
261-273-280- 301 + 310-311-501 |
||||||||||
| Tox data | 1600 mg kg −1 ( LD 50 , rat , oral ) | 1600 mg kg −1 ( LD 50 , rat , oral ) | 545 mg kg −1 ( LD 50 , rat , oral ) | ||||||||||||
presentation
Dichloroanilines may be prepared by hydrogenation of Dichlornitrobenzolen with hydrogen one using noble metal catalyst at temperatures of 80 to 180 ° C and pressures are obtained from 0.3 to 15 MPa.
use
All dichloroanilines are useful as intermediates for the preparation of drugs , dyes , pesticides and plant protection products but also flame retardants and polymers used. So z. B. 3,4-dichloroaniline into Diethofencarb and - processed into diuron via 3,4-dichlorophenyl isocyanate and 3,5-dichloroaniline into vinclozolin . Around 45,000 t of 3,4-dichloroaniline are produced worldwide per year (as of 1997).
Individual evidence
- ↑ a b c d e f g h Entry on 2,3-dichloroaniline in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c d e f g h i j Entry on 2,4-dichloroaniline in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c d e f g h i j Entry on 2,5-dichloroaniline in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c d e f g h i Entry on 2,6-dichloroaniline in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c d e f g h i j Entry on 3,4-dichloroaniline in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c d e f g h i Entry on 3,5-dichloroaniline in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ Toxicological assessment of 3,4-dichloroaniline and 2,5-dichloroaniline (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ↑ Dangers from hormonally active pesticides and biocides. ( Memento from November 9, 2012 in the Internet Archive ) (PDF; 1.1 MB)



