Vinclozolin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
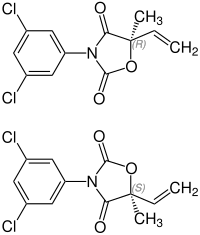
|
||||||||||||||||
| 1: 1 mixture of ( R ) -form (top) and ( S ) -form (bottom) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Vinclozolin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 9 Cl 2 NO 3 | |||||||||||||||
| Brief description |
colorless and odorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 286.11 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.51 g cm −3 |
|||||||||||||||
| Melting point |
108 ° C |
|||||||||||||||
| boiling point |
131 ° C (7 Pa ) |
|||||||||||||||
| Vapor pressure |
1.6 · 10 −5 Pa (20 ° C) |
|||||||||||||||
| solubility |
slightly soluble in water, soluble in acetone and chloroform |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Vinclozolin is a mixture of two chemical compounds ( racemate ) from the group of chlorinated nitrogen - oxygen - heterocycles with keto group and an oxazolidine - derivative (Dichlorphenyloxazolidindion). The 1: 1 mixture of the ( R ) and ( S ) forms is used as a fungicide , but the approval of vinclozolin-containing pesticides in Germany has been withdrawn. Vinclozolin is considered an endocrine disruptor .
Extraction and presentation
Vinclozolin can be synthesized starting from 3,5-dichloroaniline , whereby an oxazolidinedione (oxazolidine derivative) is added.
use
The active ingredient was used in Germany as a fungicide (e.g. in viticulture, in strawberries, cherries, lettuce, beans and rape, e.g. against gray mold , white stalk and peak drought ) in other countries also in hops and in fruit growing.
Admission
In the USA, vinclozolin was applied for by BASF in 1978 and approved in 1981. In Germany, the approval granted in 1984 was revoked at the end of 2001 (residual quantities could be used until the beginning of 2004), but residues of these are still detected in imported food. In Switzerland there were approvals for Vinclozolin against white stalk in rapeseed, individual fungal diseases in apples and apricots and against gray mold in general.
Today, the EU states including Germany and Austria as well as Switzerland are no longer permitted any pesticides containing vinclozolin.
safety instructions
Vinclozolin is toxic to reproduction, influences the hormonal system ( anti-androgenic effect) and is suspected of being carcinogenic. In addition, Synkanzerogenese proven.
The permitted daily dose is 0.005 and the acute reference dose 0.06 milligrams per kilogram of body weight and day.
Derivatives
Related pesticides (dichlorophenyldicarboximide) are:
- Chlozolinate C 13 H 11 Cl 2 NO 5
- Dichlozolin C 11 H 9 Cl 2 NO 3 , CAS number: 24201-58-9
- Iprodione C 13 H 13 Cl 2 N 3 O 3
- Isovaledione C 14 H 14 Cl 2 N 2 O 3 , CAS number: 70017-93-5
- Myclozolin C 12 H 11 Cl 2 NO 4 , CAS number: 54864-61-8
- Procymidone C 13 H 11 Cl 2 NO 2
Individual evidence
- ↑ a b c d Joint Meeting on Pesticide Residues (JMPR), Monograph for Vinclozolin , accessed December 9, 2014.
- ↑ a b c Entry on Vinclozolin in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Donald Mackay , Wan Ying Shiu, Kuo-Ching Ma, Sum Chi Lee: Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, Vol IV Nitrogen and Sulfur Containing Compounds and Pesticides , CRC Press Taylor & Francis Group 2006, ISBN 978 -1-56670-687-2 , p. 4108.
- ↑ Entry on N-3,5-dichlorophenyl-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturer or distributors can expand the harmonized classification and labeling .
- ↑ Vinclozolin data sheet from Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
- ↑ Temporary Tolerance & EUP for Use on Strawberries ( Memento of April 8, 2008 in the Internet Archive ).
- ↑ Pesticide Reregistration (PDF; 31 kB).
- ↑ Pesticide residues in carrots | Nds. State Office for Consumer Protection and Food Safety. Retrieved February 24, 2019 .
- ^ Prohibited pesticides in German vegetables. Greenpeace , November 24, 2005, accessed December 29, 2017 .
- ↑ a b Directorate-General for Health and Food Safety of the European Commission: entry on Vinclozolin in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 11, 2016.
- ↑ Extract from Environment and Health of the Federal Environment Agency (PDF; 3.3 MB).
- ↑ Determination of maximum amounts taking into account children using the example of Vinclozolin by the Federal Institute for Risk Assessment (PDF; 87 kB).


