Diethofencarb
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
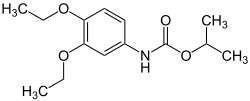
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Diethofencarb | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 21 NO 4 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 267.32 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
100 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water (26.6 mg l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Diethofencarb is a chemical compound from the group of the N- phenylcarbamates and a fungicide introduced by Sumitomo Chemical .
Extraction and presentation
Diethofencarb can be prepared starting from 3,4-dichloroaniline . This reacts with sodium ethoxide and isopropyl chloroformate ( phosgene + isopropanol ) to form the end product.
use
Diethofencarb is used as a systemic fungicide with a protective and curative effect against gray mold ( Botrytis cinerea ) z. B. used in viticulture and vegetable growing. Diethofencarb also detects benzimidazole-resistant strains.
Admission
In Germany, Austria and Switzerland, pesticides with Diethofencarb as an active ingredient are not permitted.
Individual evidence
- ↑ a b c d Entry on Diethofencarb. In: Römpp Online . Georg Thieme Verlag, accessed on December 12, 2014.
- ↑ a b Datasheet Diethofencarb at Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 78 ( limited preview in Google Book search).
- ↑ Benzimidazole fungicides such as benomyl and carbendazim.
- ↑ Basil N. Ziogas, Sobhy M. Girgis: Cross-resistance relationships between benzimidazole fungicides and diethofencarb in Botrytis cinerea and their genetical basis in Ustilago maydis . In: Pesticide Science . tape 39 , no. 3 , 1993, p. 199–205 , doi : 10.1002 / ps.2780390306 ( PDF ).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Diethofencarb in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 7, 2019.
