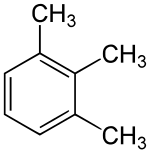
Hemellitol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Hemellitol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 12 | ||||||||||||||||||
| Brief description |
colorless liquid with an aromatic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 120.19 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.89 g cm −3 |
||||||||||||||||||
| Melting point |
−25 ° C |
||||||||||||||||||
| boiling point |
176 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.513 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Hemellitol is a chemical compound from the group of methyl-substituted benzenes , more precisely the trimethylbenzenes . The common name Hemellitol refers to the hemimellitic acid (benzene-1,2,3-tricarboxylic acid), which contains half as many (Greek hemi) carboxy groups as the mellitic acid .
Occurrence
Hemellitol occurs naturally in the form of Centaurium erythraea . The compound is a component of mineral oil and heavy oil fractions .
Extraction and presentation
Hemellitol can be obtained by reacting 2,3-dimethylbenzyl alcohol with a Grignard reagent . The compound was first synthesized by Oscar Jacobsen in 1882 .
properties
Hemellitol is a colorless oily liquid with an aromatic odor that is practically insoluble in water.
use
Hemellitol is used as an intermediate in the manufacture of fragrances. The compound is used in particular as a solvent in adhesives , but is also found in many construction products.
safety instructions
The vapors of Hemellitol can form an explosive mixture with air ( flash point 51 ° C, ignition temperature 470 ° C).
Web links
- Entry to Hemellitol . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed December 12, 2018.
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on Hemellitol in the GESTIS substance database of the IFA , accessed on December 11, 2018(JavaScript required) .
- ^ Robert A. Lewis: Hawley's Condensed Chemical Dictionary . John Wiley & Sons, 2016, ISBN 978-1-119-26784-3 , pp. 702 ( limited preview in Google Book search).
- ↑ Data sheet 1,2,3-Trimethylbenzene, analytical standard from Sigma-Aldrich , accessed on December 11, 2018 ( PDF ).
- ^ S. Gangolli, Royal Society of Chemistry (Great Britain): The Dictionary of Substances and Their Effects . Royal Society of Chemistry, 1999, ISBN 978-0-85404-838-0 , pp. 905 ( limited preview in Google Book search).
- ↑ Albert Gossauer: Structure and reactivity of biomolecules an introduction to organic chemistry . John Wiley & Sons, 2006, ISBN 3-906390-29-2 , pp. 160 ( limited preview in Google Book Search).
- ↑ Igor Jerković, Dajana Gašo-Sokač, Hrvoje Pavlović, Zvonimir Marijanović, Mirko Gugić, Ivana Petrović, Spomenka Kovač: Volatile organic compounds from Centaurium erythraea Rafn (Croatia) and the antimicrobial potential of its essential oil. In: Molecules . 17, 2012, pp. 2058-2072, PMID 22349896 .
- ^ Heinz-Gerhard Franck, Jürgen W. Stadelhofer: Industrial Aromatic Chemistry Raw Materials Processes Products . Springer-Verlag, 2013, ISBN 978-3-662-07875-4 , pp. 301 ( limited preview in Google Book search).
- ↑ a b Lee Irvin Smith, Leo J. Spillane: Polyalkylbenzenes. XXXI. Preparation and Physical Properties of 1,2,3-Trimethylbenzene (Hemimellithene). In: Journal of the American Chemical Society . 62, 1940, pp. 2639-2642, doi : 10.1021 / ja01867a017 .
- ↑ Oscar Jacobsen: About the Hemellithol. In: Reports of the German Chemical Society . 19, 1886, p. 2517, doi : 10.1002 / cber.188601902197 .
- ↑ Entry on 1,2,3-trimethylbenzene in the Hazardous Substances Data Bank , accessed December 11, 2018.
- ↑ Enius: 1,2,3 trimethylbenzene , accessed December 11, 2018.

