Methyl substituted benzenes
| Methyl substituted benzenes | |||
| benzene |
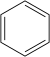
|
||
| 6 ° C | |||
| 80 ° C | |||
|
Toluene C 6 H 5 (CH 3 ) |

|
||
| −95 ° C | |||
| 111 ° C | |||
|
Dimethylbenzenes C 6 H 4 (CH 3 ) 2 |
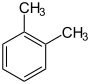 |
 |
|
| o -xylene | m -xylene | p -xylene | |
| −25 ° C | −48 ° C | 13 ° C | |
| 144 ° C | 139 ° C | 138 ° C | |
|
Trimethylbenzenes C 6 H 3 (CH 3 ) 3 |
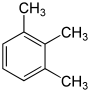 |
 |

|
| Hemellitol | Pseudocumene | Mesitylene | |
| −25 ° C | −44 ° C | −45 ° C | |
| 176 ° C | 169 ° C | 165 ° C | |
|
Tetramethylbenzenes C 6 H 2 (CH 3 ) 4 |
 |
 |

|
| Prehnitol | Isodurol | Durol | |
| −6.3 ° C | −20 ° C | 79.2 ° C | |
| 205 ° C | 197.9 ° C | 196.8 ° C | |
|
Pentamethylbenzene C 6 H (CH 3 ) 5 |
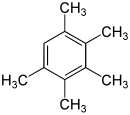
|
||
| 49-51 ° C | |||
| 231 ° C | |||
|
Hexamethylbenzene C 6 (CH 3 ) 6 |
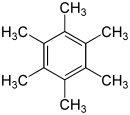
|
||
| 165.5 ° C | |||
| 263.4 ° C | |||
The methyl-substituted benzenes are derived from benzene , in which one or more hydrogen atoms are replaced by a methyl group . This results in 12 different compounds that differ from one another in terms of degree of substitution and symmetry. These properties play the main role here and are shown in comparison.
A distinction is made according to the number of methyl groups and their position on the ring:
- Toluene C 6 H 5 (CH 3 )
- Xylenes C 6 H 4 (CH 3 ) 2 , three structural isomers (1,2-xylene, 1,3-xylene, 1,4-xylene)
- Trimethylbenzenes C 6 H 3 (CH 3 ) 3 , three structural isomers (hemellitol, pseudocumene, mesitylene )
- Tetramethylbenzenes C 6 H 2 (CH 3 ) 4 , three structural isomers (Prehnitol, Isodurol, Durol )
- Pentamethylbenzene C 6 H (CH 3 ) 5
- Hexamethylbenzene C 6 (CH 3 ) 6
properties
Boiling points
Overall, the boiling points increase on average with each methyl group added by about 30 ° C (80 - 111 - ø 140 - ø 170 - ø 200 - 231 - 263). The boiling points of the three isomers of the di-, tri- and tetramethylbenzenes are close to each other and differ within a group by a maximum of 11 ° C (mesitylene / hemellitol). The symmetry does not play a special role here.
Melting points
With the melting points, the symmetry is particularly important. First of all, starting from benzene to toluene, the melting point drops significantly by around 100 ° C from +6 to −95 ° C - due to the introduction of a single methyl group into the highly symmetrical benzene molecule.
Among the di-, tri- and tetramethylbenzenes, p- xylene ( C 2 ), mesitylene ( C 3 ) and durol ( C 2 ) are the representatives with the highest symmetry.
- Dimethylbenzenes: The p -Xylene, which has the highest symmetry, has the highest melting point of 13 ° C.
- Trimethylbenzenes: The melting points behave rather inconsistently compared to the xylenes. The melting point changes only insignificantly from o -xylene to hemellitol, and only slightly from m -xylene to pseudocumene. Mesitylene is unusual . The most highly symmetrical representative in this group has a melting point of −45 ° C and is therefore the lowest of all three isomers.
- Tetramethylbenzenes: Due to its symmetry, Durol has the highest melting point of 79.2 ° C; in contrast to the other two isomers, it is a solid.
- Pentamethylbenzene is a solid with a melting point of 49-51 ° C. In comparison with the tetramethylbenzenes, this is lower than that of Durol at 79.2 ° C due to the lower symmetry. However, it is also higher than that of Isodurol at −20 ° C and Prehnitol at −6.3 ° C due to the higher degree of substitution.
- Hexamethylbenzene is a solid; the melting point is 165.5 ° C and is thus the highest of the methyl-substituted benzenes.
density
Starting from the benzene, the density increases only slightly and irregularly (0.88 - 0.87 - 0.88 / 0.86 / 0.86 - 0.89 / 0.88 / 0.87 - - / 0 , 89 / 0.84 - 0.92 - 1.04).
Individual evidence
- ↑ a b c d Entry on benzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b c d Entry on toluene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on o-xylene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on m-xylene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on p-xylene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on Hemellitol in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on pseudocumene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on mesitylene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b Entry on Isodurol in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on Durol in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b c d data sheet pentamethylbenzene from Sigma-Aldrich , accessed on December 28, 2019 ( PDF ).
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-280.
literature
- Allinger , Cava , de Jongh , Johnson , Lebel , Stevens : Organic Chemistry , 1st Edition, Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X , pp. 18-19, 367-368.
- Streitwieser / Heathcock : Organic Chemistry , 1st edition, Verlag Chemie, Weinheim 1980, ISBN 3-527-25810-8 , pp. 1073-1080.
- Beyer / Walter : Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 439-444.
- Morrison / Boyd : Textbook of Organic Chemistry , 3rd Edition, Verlag Chemie, Weinheim 1986, ISBN 3-527-26067-6 , pp. 709-712.
- HW Earhart, Andrew P. Komin: Polymethylbenzenes . In: Kirk-Othmer Encyclopedia of Chemical Technology , 2000, doi : 10.1002 / 0471238961.1615122505011808.a01 .