Bromodihydroxybenzenes
The bromodihydroxybenzenes form a group of aromatic compounds which are derived from phenol or dihydroxybenzenes as well as from bromobenzene . The structure consists of a benzene ring with two attached hydroxyl groups (-OH) and a bromine atom (-Br) as substituents . Their different arrangement results in six constitutional isomers with the empirical formula C 6 H 5 BrO 2 .
Uniform naming is difficult because the dihydroxybenzenes do not form a common common name. They can therefore be understood as derivatives of catechol (2 isomers), resorcinol (3 isomers) and hydroquinone (1 isomer). Conversely, they can be viewed as dihydroxy derivatives of bromobenzene. Since the phenolic component predominates here, bromodihydroxybenzenes , i.e. derivatives of dihydroxybenzenes, are more obvious here.
| Bromodihydroxybenzenes | ||||||||||||
| Surname |
3-bromopyrocatechol , 1,2-dihydroxy- 3-bromobenzene |
4-bromopyrocatechol , 1,2-dihydroxy- 4-bromobenzene |
2-bromoresorcinol , 1,3-dihydroxy- 2-bromobenzene |
4-bromoresorcinol , 1,3-dihydroxy- 4-bromobenzene |
5-bromoresorcinol , 1,3-dihydroxy- 5-bromobenzene |
Bromohydroquinone , 1,4-dihydroxy- 2-bromobenzene |
||||||
| Structural formula |
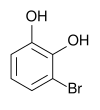
|
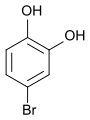
|

|

|
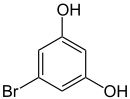
|
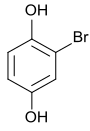
|
||||||
| CAS number | 14381-51-2 | 17345-77-6 | 6751-15-9 | 6626-15-9 | 106120-04-1 | 583-69-7 | ||||||
| PubChem | 26659 | 28487 | 604810 | 81105 | 557383 | 68502 | ||||||
| Molecular formula | C 6 H 5 BrO 2 | |||||||||||
| Molar mass | 189.01 g mol −1 | |||||||||||
| Physical state | firmly | |||||||||||
| Melting point | 39-42 ° C | 87 ° C | 102-103 ° C | 99-102 ° C | 112-116 ° C | |||||||
| boiling point | 118–120 ° C (12 hPa ) | 150 ° C (16 hPa) | ||||||||||
| pK s value | 9.40 |
|
|
|||||||||
|
GHS labeling |
|
|
|
|
|
|
||||||
| H and P phrases | 315-319-335 | see above | see above | 302-312-315-319-332-335 | see above | 315-319-335 | ||||||
| no EUH phrases | see above | see above | no EUH phrases | see above | no EUH phrases | |||||||
|
261-302 + 352 305 + 351 + 338-321-405-501 |
see above | see above | 261-280-305 + 351 + 338 | see above | 261-305 + 351 + 338 | |||||||
Individual evidence
- ↑ a b Data sheet 3-Bromocatechol from AlfaAesar, accessed on August 15, 2010 ( PDF )(JavaScript required) .
- ↑ a b c d e f g Dictionary of organic compounds , p. 932 ( limited preview in Google book search).
- ↑ a b Data sheet 4-Bromoresorcinol, 98% from Acros, accessed on February 22, 2010.
- ^ Entry on Bromhydroquinone at ChemicalBook , accessed on September 19, 2011.
- ^ Howard S. Mason: "The allergenic Principles of Poison Ivy. VI. Note on the Synthesis of 3-Substituted Catechols ”, in: J. Am. Chem. Soc. , 1947 , 69 (9), pp. 2241-2242; doi : 10.1021 / ja01201a514 .
- ↑ Data sheet 4-bromoresorcinol from Sigma-Aldrich , accessed on March 18, 2011 ( PDF ).
- ↑ Datasheet Bromohydroquinone from Sigma-Aldrich , accessed on March 14, 2011 ( PDF ).
