3-bromopyrocatechol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3-bromopyrocatechol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 5 BrO 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 189.01 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
39-42 ° C |
||||||||||||||||||
| boiling point |
118–120 ° C (12 h Pa ) |
||||||||||||||||||
| pK s value |
9.40 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
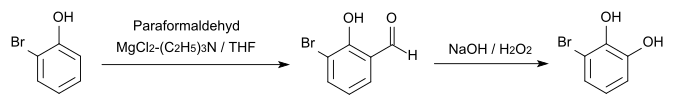
3-bromopyrocatechol is a chemical compound that belongs to the group of phenols . It is one of the two positionally isomeric monobromo derivatives of catechol (1,2-hydroxybenzene) alongside 4-bromopychol . It crystallizes in needles.
presentation
3-bromopyrocatechol can be obtained from 2,3- dimethoxybromobenzene , which can be obtained from veratrole , by reacting it with aluminum chloride in chlorobenzene .
The synthesis from 2- bromophenol is also possible.
Analytical evidence
For qualitative analytical proof, the bromination with potassium bromide and bromine produces the tetrabromo derivative , which has a melting point of 192 ° C.
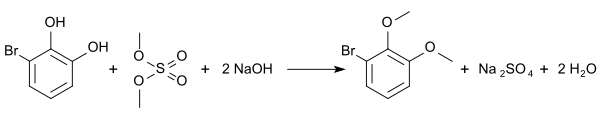
Simple methylation with dimethyl sulfate leads to 2-bromo-6-methoxyphenol (6- bromoguajacol , CAS number: 28165-49-3), the melting point of which is 63 ° C.
Complete methylation leads to 1-bromo-2,3-dimethoxybenzene (3- bromveratrol , CAS number: 5424-43-1), which has a boiling point of 114 ° C at 5 mm Hg.
Individual evidence
- ↑ a b c data sheet 3-bromopyrocatechol from AlfaAesar, accessed on April 30, 2017 ( PDF )(JavaScript required) .
- ^ A b c Howard S. Mason: "The allergenic Principles of Poison Ivy. VI. Note on the Synthesis of 3-Substituted Catechols", in: J. Am. Chem. Soc. , 1947 , 69 (9), pp. 2241-2242; doi: 10.1021 / ja01201a514 .
- ↑ a b c Dictionary of organic compounds , p. 932 ( limited preview in Google book search).
- ↑ TV Hansen, L. Skattebøl: "One-pot synthesis of substituted catechols from the corresponding phenols", in: Tetrahedron Letters , 2005 , 46 (19), pp. 3357-3358; doi: 10.1016 / j.tetlet.2005.03.082 .
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 331.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 653.