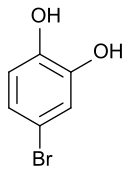
4-bromopyrocatechol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4-bromopyrocatechol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 5 BrO 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 189.01 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
87 ° C |
||||||||||||||||||
| pK s value |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4-bromopyrocatechol is a chemical compound that belongs to the group of phenols . Along with 3-bromopyrocatechol, it is one of the two positionally isomeric monobromo derivatives of catechol (1,2-hydroxybenzene).
presentation
4-Bromo catechol can be made by bromination of catechol with pyridine dibromide bromohydrate in glacial acetic acid .
Analytical evidence
For qualitative analytical proof, the bromination with potassium bromide and bromine produces the tetrabromo derivative , which has a melting point of 192 ° C.
Derivatives
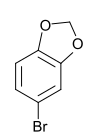
The methyl ethers of 4-bromopyrocatechol are listed in the table below:
| Methyl ether of 4-bromopyrocatechol | ||||
| Names | 5-bromo-2-methoxyphenol 5-bromoguaiacol |
4-bromo-2-methoxyphenol 4-bromoguaiacol |
4-Bromo-1,2-Dimethoxybenzene 4-Bromveratrol |
5-Bromo-1,3-benzodioxole 4-Bromo-1,2- (methylenedioxy) benzene |
| Structural formula | 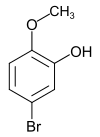 |
 |
 |
|
| CAS number | 37942-01-1 | 7368-78-7 | 2859-78-1 | 2635-13-4 |
| Molecular formula | C 7 H 7 O 2 Br | C 8 H 9 O 2 Br | C 7 H 8 O 2 Br | |
| Molar mass | 203.0 g mol −1 | 217.0 g mol −1 | 201.0 g mol −1 | |
| Physical state | firmly | liquid | ||
| Melting point | 65 ° C | 46 ° C | ||
| boiling point | 150 ° C (20 mbar) |
181–182 ° C (60 mbar) |
255–256 ° C 157–158 ° C (30 mbar) |
226–228 ° C 103–107 ° C (10 mbar) |
The esterification with acetic anhydride produces the diacetate (CAS number: 66373-95-3), which has a melting point of 109 ° C.
Individual evidence
- ↑ a b c d e Dictionary of organic compounds , p. 932 ( limited preview in Google book search).
- ↑ There is not yet a harmonized classification for this substance . A labeling of 4-bromopyrocatechol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on December 28, 2019, is reproduced from a self-classification by the distributor .
- ↑ a b Safety Data Sheet 4-Bromocatechol. (PDF) Apollo Scientific Limited, April 4, 2017, accessed March 5, 2020 .
- ↑ KW Rosenmund, W. Kuhnhenn: About a method for the bromination of organic compounds , in: Reports of the German Chemical Society , 1923 , 56 (6), pp. 1262-1269; doi : 10.1002 / cber.19230560605 .
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 331.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 653.

