4-bromoresorcinol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
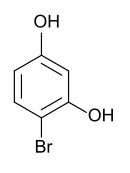
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4-bromoresorcinol | ||||||||||||||||||
| other names |
4-bromobenzene-1,3-diol |
||||||||||||||||||
| Molecular formula | C 6 H 5 BrO 2 | ||||||||||||||||||
| Brief description |
pink crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 189.01 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
99-102 ° C |
||||||||||||||||||
| boiling point |
150 ° C (16 hPa) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4-Bromoresorcinol is a chemical compound that belongs to the group of phenols . Along with 2-bromoresorcinol and 5-bromoresorcinol, it is one of the three positionally isomeric monobromo derivatives of resorcinol (1,3-dihydroxybenzene).
presentation
4-bromoresorcinol can be prepared from 2,4-dihydroxybenzoic acid via 5-bromo-2,4-dihydroxybenzoic acid as an intermediate. 2,4-Dihydroxybenzoic acid can be obtained from resorcinol by a Kolbe-Schmitt reaction with potassium hydrogen carbonate .
Another synthetic route is from resorcinol via 4,6-dibromoresorcinol, from which a bromine atom is removed again with sodium sulfate / sodium hydroxide .
Reactions
The introduction of an acetyl group provides 1- (5-bromo-2,4-dihydroxyphenyl) -ethanone (CAS number: 60965-25-5), and can either by a Nencki reaction with zinc chloride or by Fries rearrangement of the diacetate with Aluminum chloride can be carried out.
4-bromoresorcinol is a synthetic building block for the preparation of 6-bromo-substituted coumarins - z. B. is formed by a condensation reaction with acetoacetic ester 6-bromo-7-hydroxy-4-methylcoumarin.
The reaction with malic acid also produces 6-bromo-7-hydroxycoumarin.
Individual evidence
- ↑ a b c data sheet 4-bromoresorcinol at Acros, accessed on February 22, 2010.
- ↑ a b Data sheet 4-bromoresorcinol from Sigma-Aldrich , accessed on March 18, 2011 ( PDF ).
- ↑ RB Sandin, RA McKee: 4-Bromoresorcinol In: Organic Syntheses . 17, 1937, p. 23, doi : 10.15227 / orgsyn.017.0023 ; Coll. Vol. 2, 1943, p. 100 ( PDF ).
- ↑ M. Kidney Stone, DA Clibbens: β-Resorcylic Acid In: Organic Syntheses . 10, 1930, p. 94, doi : 10.15227 / orgsyn.010.0094 ; Coll. Vol. 2, 1943, p. 557 ( PDF ).
- ↑ E. Kiehlmann, RW Lauener: Bromophloroglucinols and their methyl ethers . In: Canadian Journal of Chemistry . 67, 1989, pp. 335-344, doi : 10.1139 / v89-055 .
- ↑ a b R. Martin: Handbook of Hydroxyacetophenones Set: Preparation and Physical Properties . Springer Verlag, 2005, ISBN 1-4020-2290-5 , pp. 26 ( limited preview in Google Book search).
- ^ The Zebrafish: Genetics, Genomics and Informatics: Genetics, Genomics and ... Academic Press, 2004, ISBN 0-08-052251-3 , pp. 161 ( limited preview in Google Book search).
- ↑ §Author§: Indian Journal of Chemistry . Council of Scientific & Industrial Research, 2009, ISBN 0-470-18832-4 , p. 262 ( limited preview in Google Book search).





