2-bromoresorcinol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-bromoresorcinol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 5 BrO 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 189.01 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
102-103 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2-Bromoresorcinol is an aromatic chemical compound that belongs to the group of phenols . Along with 4-bromoresorcinol and 5-bromoresorcinol, it is one of the three positionally isomeric monobromo derivatives of resorcinol (1,3-dihydroxybenzene).
presentation
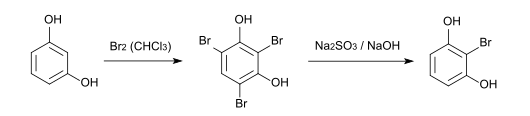
2-bromoresorcinol can be produced from resorcinol via the intermediate 2,4,6-tribromoresorcinol , which is formed during the complete bromination with bromine in chloroform . The bromine atoms in positions 4 and 6 are then removed by reaction with sodium sulfite and sodium hydroxide in a 5: 1 water / methanol mixture.
The synthesis from 2-aminoresorcinol by a Sandmeyer reaction fails because the diazotization of 2-aminoresorcinol gives 4-nitroso-2-diazoresorcinol.
Historically, 2-bromoresorcinol was produced in several stages starting from 2,4-dihydroxybenzoic acid. This was first nitrated to 5-nitro-2,4-dihydroxybenzoic acid and then reacted with elemental bromine in glacial acetic acid to give 3-bromo-5-nitro-2,4-dihydroxybenzoic acid. Reduction of this acid with tin (II) chloride and hydrochloric acid leads to the corresponding amino compound. This is diazotized with sodium nitrite and hydrochloric acid and then converted to 3-bromo-2,4-dihydroxybenzoic acid by boiling the diazonium salt formed. Decarboxylation of this acid eventually leads to 2-bromoresorcinol.
Reactions
The introduction of an acetyl group provides 1- (3-bromo-2,4-dihydroxyphenyl) ethanone (CAS number: 60990-39-8) and can be carried out by a Nencki reaction with zinc chloride .
2-Bromoresorcinol is a synthetic building block for the preparation of substituted coumarins , - z. B. is formed in a condensation reaction with ethyl benzoylacetate 8-bromo-7-hydroxy-4-phenylcoumarin.
Individual evidence
- ^ Dictionary of organic compounds , p. 932 ( limited preview in Google book search).
- ↑ a b Data sheet 2-bromoresorcinol from Sigma-Aldrich , accessed on February 16, 2020 ( PDF ).
- ↑ Dissertation: Concave N-Heterocyclic Catalyst Systems , Timo Liebig, University of Kiel, 2006, urn : nbn: de: gbv: 8-diss-17594 .
- ↑ a b R.F. Milligan, FJ Hope: "The Preparation of 2-Chlororesorcinol" in J. Am. Chem. Soc. 1941 , 63 (2), p. 544. doi : 10.1021 / ja01847a052
- ↑ F. v. Hemmelmayr: "About some new derivatives of dioxybenzoic acids" in :months booklet for chemistry , 1912 , 33 , pp. 971-998; doi : 10.1007 / BF01552742 .
- ↑ a b F. v. Hemmelmayr: "About some new derivatives of di- and trioxybenzoic acids", in: Monatshefte für Chemie , 1914 , 35 , pp. 1-8; doi : 10.1007 / BF01519727 .
- ^ GP Rice: "The Orientation of the Bromine Atom in Bromodimethoxybenzoic acid" in J. Am. Chem. Soc. 1926 , 48 (12), pp. 3125-3130. doi : 10.1021 / ja01691a017
- ^ R. Martin: Handbook of Hydroxyacetophenones Set: Preparation and Physical Properties . Springer Verlag, 2005, ISBN 1-4020-2290-5 , pp. 25 ( limited preview in Google Book search).
- ↑ KR Shah, KN Trivedi: "Studies in the synthesis of furocoumarins. XXIII. Synthesis of substituted psoralenes from 2-bromoresorcinol." in Australian Journal of Chemistry 1974 , 27 (9), pp. 1971-1976. doi : 10.1071 / CH9741971