Nitrotoluidines
In chemistry, the nitrotoluidines are a group of substances that are derived from nitrobenzene , toluene (methylbenzene) and aniline (aminobenzene). The structure consists of a benzene ring with attached nitro (–NO 2 ), methyl (–CH 3 ) and amino (–NH 2 ) groups as substituents . Their different arrangement results in ten constitutional isomers with the empirical formula C 7 H 8 N 2 O 2 .
If one proceeds from the disubstituted parent compounds, then one can regard the nitrotoluidines as methyl derivatives of nitroanilines , as amino derivatives of nitrotoluenes or as nitro derivatives of toluidines . The naming followed the last variant because the toluidines are the only ones of the three disubstituted parent compounds that form a completely separate name.
Nitrotoluidines can be obtained by nitration of acetyltoluidines with mixed acid and subsequent hydrolysis (e.g. 2-nitro- p -toluidine from N -acetyl- p -toluidine ). They are used as intermediate products in the manufacture of azo dyes . Thus, 2-nitro- p -toluidine serves as the diazo component in C.1. Pigment Yellow 1 and C.1. Pigment Red 3. 5-Nitro-o-toluidine must not be released after reductive cleavage of azo groups from textiles or leather products that come into direct contact with human skin for a long period of time (Appendix 1 of the Consumer Goods Ordinance ).
| Nitrotoluidines | ||||||||||||
| Trunk connection |

|
|||||||||||
| o -Toluidine | ||||||||||||
| Structural formula |

|

|

|

|
||||||||
| Surname |
|
|
|
|
||||||||
| CAS number | 603-83-8 | 99-52-5 | 99-55-8 | 570-24-1 | ||||||||
| ECHA InfoCard | 100.009.145 | 100.002.511 | 100.002.514 | 100,008,482 | ||||||||
| PubChem | 11783 | 7441 | 7444 | 11298 | ||||||||
| Molecular formula | C 7 H 8 N 2 O 2 | |||||||||||
| Molar mass | 152.16 g mol −1 | |||||||||||
| Physical state | firmly | |||||||||||
| Brief description | yellow odorless powder |
yellow odorless powder | ||||||||||
| Melting point | 88-90 ° C | 131-133 ° C | 107-108 ° C | 93-96 ° C | ||||||||
| boiling point | 305 ° C | > 300 ° C (decomposition) | > 130 ° C (decomposition) | |||||||||
| density | 1.365 g cm −3 | |||||||||||
| solubility | practically insoluble in water |
|||||||||||
|
GHS labeling |
|
|
|
|||||||||
| H and P phrases | 301-311-331-373-411 | 301-311-331-351-412 | 301-311-331-373-411 | |||||||||
| 261-273-280-301 + 310-311 | ||||||||||||
| Nitrotoluidines | |||||||
| Trunk connection |

|
||||||
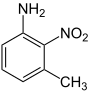
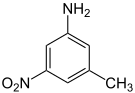
| m -toluidine | |||||||
| Structural formula |
|

|
|

|
|||
| Surname |
|
|
|
|
|||
| CAS number | 601-87-6 | 611-05-2 | 618-61-1 | 578-46-1 | |||
| ECHA InfoCard | 100.009.317 | 100.008.568 | |||||
| PubChem | 69038 | 11898 | 3565037 | 11356 | |||
| Molecular formula | C 7 H 8 N 2 O 2 | ||||||
| Molar mass | 152.16 g mol −1 | ||||||
| Physical state | firmly | ||||||
| Brief description | yellow red powder | orange powder | |||||
| Melting point | 131-136 ° C | 110-111 ° C | |||||
| boiling point | |||||||
| density | |||||||
| solubility | soluble in tetrahydrofuran | ||||||
|
GHS labeling |
|
||||||
| H and P phrases | 301-311-331-373-411 | ||||||
| ? | ? | ? | 261-273-280-301 + 310-311 | ||||
| Nitrotoluidines | |||||
| Trunk connection |
|
||||
| p -toluidine | |||||
| Structural formula |

|

|
|||
| Surname |
|
|
|||
| CAS number | 89-62-3 | 119-32-4 | |||
| ECHA InfoCard | 100.001.750 | 100.003.922 | |||
| PubChem | 6978 | 8390 | |||
| Molecular formula | C 7 H 8 N 2 O 2 | ||||
| Molar mass | 152.16 g mol −1 | ||||
| Physical state | firmly | ||||
| Brief description | orange powder with a characteristic odor |
||||
| Melting point | 116 ° C | 74-77 ° C | |||
| boiling point | 210 ° C (decomposition) | ||||
| density | 1.31 g cm −3 | ||||
| solubility |
|
||||
|
GHS labeling |
|
||||
| H and P phrases | 301-311-331-373-411 | ||||
| 261-273-280-301 + 310-311 | |||||
Individual evidence
- ↑ a b c Toxicological assessment of 2-nitro-4-methylaniline (PDF) from the trade association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ↑ a b c d Entry on 4-nitro-o-toluidine in the GESTIS substance database of the IFA , accessed on September 27, 2019(JavaScript required) .
- ↑ a b c d Entry on 5-nitro-o-toluidine in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b c data sheet 2-Methyl-3-nitroaniline from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ a b Data sheet 2-Methyl-6-nitroaniline from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ 2-Methyl-4-nitroaniline data sheet from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ Data sheet 2-Methyl-5-nitroaniline from Sigma-Aldrich , accessed on December 27, 2019 ( PDF ).
- ↑ a b Entry on 4-nitro-m-toluidine at TCI Europe, accessed on December 27, 2019.
- ↑ a b Data sheet 5-Methyl-2-nitroaniline from Sigma-Aldrich , accessed on December 27, 2019 ( PDF ).
- ↑ Entry on nitrotoluidine, isomers in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b c d Entry on 2-nitro-p-toluidine in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ Entry on 3-nitro-p-toluidine in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ Data sheet 4-Methyl-2-nitroaniline from Sigma-Aldrich , accessed on December 27, 2019 ( PDF ).
- ↑ Data sheet 4-Methyl-3-nitroaniline from Sigma-Aldrich , accessed on December 27, 2019 ( PDF ).


