Tribromobenzenes
| Tribromobenzenes | |||||||
| Surname | 1,2,3-tribromobenzene | 1,2,4-tribromobenzene | 1,3,5-tribromobenzene | ||||
| other names | vic. -Tribromobenzene | asym. -tribromobenzene | sym. -tribromobenzene | ||||
| Structural formula |

|

|

|
||||
| CAS number | 608-21-9 | 615-54-3 | 626-39-1 | ||||
| PubChem | 11842 | 12002 | 12279 | ||||
| Molecular formula | C 6 H 3 Br 3 | ||||||
| Molar mass | 314.80 g mol −1 | ||||||
| Physical state | firmly | ||||||
| Melting point | 87-88 ° C | 44.5 ° C | 122.8 ° C | ||||
| boiling point | 275 ° C | 271 ° C | |||||
| solubility | insoluble in water | ||||||
|
GHS labeling |
|
|
|
||||
| H and P phrases | see above | 315-319-335-410 | 413 | ||||
| see above | no EUH phrases | no EUH phrases | |||||
| see above | 261-273-305 + 351 + 338-501 | no P-phrases | |||||
The tribromobenzenes (according to the IUPAC tribromobenzenes ) form a group of substances in chemistry , the structure of which consists of a benzene ring with three bromine atoms (-Br) as substituents . Their different arrangement results in three constitutional isomers with the empirical formula C 6 H 3 Br 3 .
presentation
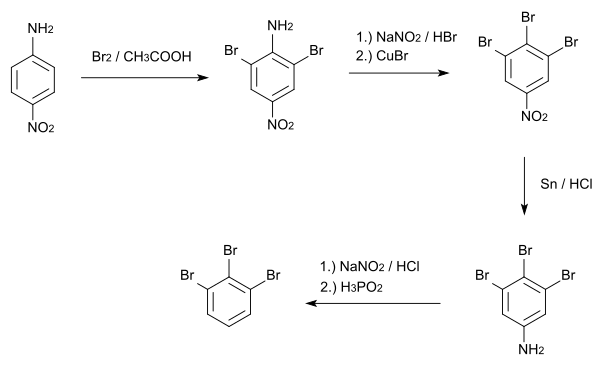
1,2,3-tribromobenzene
1,2,3-Tribromobenzene is made from p-nitroaniline , which is first brominated with elemental bromine and glacial acetic acid at positions 2 and 6. Then the amino group is replaced by a bromine atom using a Sandmeyer reaction . The 3,4,5-tribromonitrobenzene formed is then reduced with tin and hydrochloric acid to 3,4,5-tribromaniline. Finally, the amino group is removed after diazotization .
1,2,4-tribromobenzene
The starting material for the preparation of 1,2,4-tribromobenzene is acetanilide , which is first brominated at positions 2 and 4 with gaseous bromine, which is introduced into an aqueous suspension . Treatment with sodium hydroxide solution and subsequent boiling with sulfuric acid produces 2,4-dibromaniline. The amino group can then be replaced by a bromine atom by means of a Sandmeyer reaction.
1,3,5-tribromobenzene
1,3,5-Tribromobenzene is made from aniline , which is converted with elemental bromine in hydrochloric acid to 2,4,6-Tribromaniline . This compound is then diazotized and reacted with sulfuric acid in ethanol to give the desired product.
properties
1,2,4-tribromobenzene
1,2,4-tribromobenzene crystallizes in needles with an aromatic odor. It is soluble in ethanol and diethyl ether , but only slightly soluble in benzene . It is practically insoluble in water (0.0101 g / l). The flash point of 1,2,4-tribromobenzene is above 110 ° C. Nitration of 1,2,4-tribromobenzene with nitric acid and sulfuric acid leads to 1,2,4-tribromo-3,5-dinitrobenzene.
1,3,5-tribromobenzene
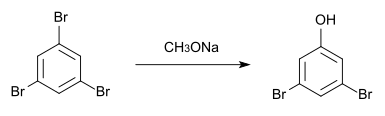
1,3,5-Tribromobenzene crystallizes in the orthorhombic crystal system with the space group P 2 1 2 1 2 1 (space group No. 19) and the lattice parameters a = 1424.4 pm , b = 1357.7 pm and c = 408.4 pm. In the unit cell there are four formula units . It is isomorphic to 1,3,5-trichlorobenzene . It is sparingly soluble in hot ethanol. It reacts with sodium methoxide at 130 ° C to form 3,5-dibromophenol .
Individual evidence
- ↑ SL Solenova, TL Khotsyanova, Yu. T. Struchkov: Steric hindrance and molecular conformation. In: Bulletin of the Academy of Sciences of the USSR Division of Chemical Science. 9, 1960, pp. 292-299, doi : 10.1007 / BF00942906 .
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-488.
- ↑ Data sheet 1,2,4-Tribromobenzene from Sigma-Aldrich , accessed on May 11, 2011 ( PDF ).
- ↑ Data sheet 1,3,5-Tribromobenzene from Sigma-Aldrich , accessed on May 11, 2011 ( PDF ).
- ↑ CL Jackson, FB Gallivan: "On the 3,4,5-Tribromaniline and some derivatives of unsymmetrical tribromobenzene", in: American Chemical Journal , 1898 , 20 , pp. 179-189; Full text .
- ^ A b C.L. Jackson, FB Gallivan: "Some derivatives of unsymmetrical tribromobenzene", in: American Chemical Journal , 1896 , 18 , pp. 238-252; Full text .
- ^ A b G. H. Coleman, WF Talbot: sym.-Tribromobenzene In: Organic Syntheses . 13, 1933, p. 96, doi : 10.15227 / orgsyn.013.0096 ; Coll. Vol. 2, 1943, p. 592 ( PDF ).
- ↑ Test specification : 1,3,5-tribromobenzene (PDF) from the collection of integrated organic-chemical internship at the University of Regensburg, accessed on October 30, 2011.
- ↑ a b Heilbron: "Dictionary of organic compounds, Volume Four", 1953 , p. 545; Full text .
- ^ IUPAC-NIST Solubility Database
- ↑ MSDS for www.chemcas.com
- ↑ A. Belaaraj, Nguyen-ba-Chanh, Y. Haget, MA Cuevas-Diarte: "Crystal data for 1,3,5-trichlorobenzene and 1,3,5-tribromobenzene at 293 K", in: J. Appl. Cryst. , 1984 , 17 , p. 211; doi : 10.1107 / S002188988401133X .