3,5-dibromophenol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3,5-dibromophenol | |||||||||||||||
| Molecular formula | C 6 H 4 Br 2 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 251.9 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
81 ° C |
|||||||||||||||
| pK s value |
8.06 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
3,5-Dibromophenol is a chemical compound that belongs to both the phenols and the halogen aromatic compounds .
presentation
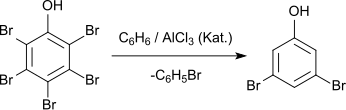
3,5-Dibromophenol can be prepared from pentabromophenol by reaction with benzene in the presence of aluminum chloride.
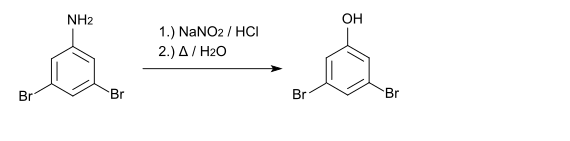
3,5-Dibromophenol can also be prepared from 3,5-dibromaniline by diazotization and subsequent boiling of the diazonium salt. The starting material for 3,5-dibromaniline is 4-nitroaniline .
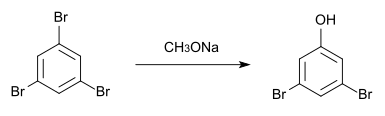
It can also be made from 1,3,5-tribromobenzene and sodium methoxide .
Derivatives
The methyl ether can be prepared by methylation with dimethyl sulfate and is also known under the common name 3,5-dibromanisole . Its melting point is 40 ° C.
The ethyl ether (3,5-dibromophenetole) boils at 268 ° C. Esterification with acetic anhydride yields the acetate, which melts at 53 ° C.
The bromination of 3,5-dibromophenol with potassium bromide and bromine leads to pentabromophenol , the melting point of which is 225 ° C.
The nitration of 3,5-dibromophenol with nitric acid and sulfuric acid leads to 3,5-dibromo-2,4,6-trinitrophenol , the melting point of which is 173 ° C.
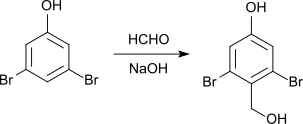
With formaldehyde and sodium hydroxide solution , 2,6-dibromo-4-hydroxybenzyl alcohol is formed with a melting point of 180 ° C.
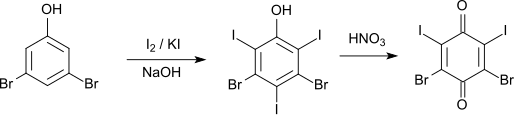
The iodination of 3,5-dibromophenol with iodine and potassium iodide in sodium hydroxide solution initially leads to 3,5-dibromo-2,4,6-triiodophenol (melting point 199 ° C). This can be converted with nitric acid to 2,4-dibromo-3,5-diiodo- p -benzoquinone.
Individual evidence
- ↑ a b c d e Dictionary of organic compounds , p. 1971 ( limited preview in the Google book search).
- ↑ a b data sheet 3,5-dibromophenol from Sigma-Aldrich , accessed on March 18, 2011 ( PDF ).
- ^ A b M. Kohn, M. Weißberg: "About m-Bromphenole. VI. Communication on Bromphenole", in: Monatshefte für Chemie , 1924 , 45 (7-8), pp. 295-303; doi : 10.1007 / BF01521913 .
- ↑ a b c M. JJ Blanksma: "Bromuration et nitration de phénols méta-substités", in: Rec. Trav. Chim. , 1907 , 27 , pp. 25-41; Full text .
- ^ MAF Holleman: "Etudes sur la formation simultanée des produits de substitution isomères du benzène. Nitration des dibromobenzènes", in: Rec. Trav. Chim. , 1906 , 25 , pp. 191-; Full text ; doi : 10.1002 / recl.19060250602 .
- ↑ Heilbron: "Dictionary of organic compounds, Volume Four", 1953 , p. 545; Full text .
- ^ M. Kohn, A. Rosenfeld: "New observations on halophenols. XIV. Communication on bromophenols", in: Monatshefte für Chemie , 1925 , 46 (1–2), pp. 101–117; doi : 10.1007 / BF01525494 .