3,5-dibromaniline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
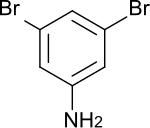
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3,5-dibromaniline | ||||||||||||||||||
| Molecular formula | C 6 H 5 Br 2 N | ||||||||||||||||||
| Brief description |
yellowish solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 250.9 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.9556 g cm −3 (16 ° C) |
||||||||||||||||||
| Melting point |
54 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
3,5-Dibromaniline is a chemical compound that belongs to both the anilines and the halogen aromatic compounds .
presentation
3,5-Dibromaniline is prepared from 4-nitroaniline , which is first brominated with hydrochloric acid and elemental bromine in positions 2 and 6. Then it is deaminated to 3,5-dibromonitrobenzene and the end product is finally synthesized by reduction with iron and dilute sulfuric acid.
Reactions
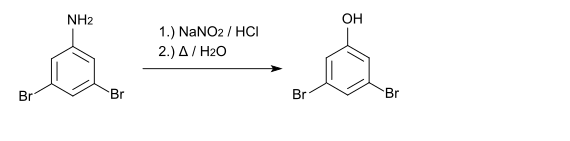
With sodium nitrite and hydrochloric acid , a diazonium compound is formed which can be converted to 3,5-dibromophenol by boiling .
Individual evidence
- ↑ a b c d e Entry on 3,5-dibromaniline at TCI Europe, accessed on July 26, 2015.
- ^ MAF Holleman: "Etudes sur la formation simultanée des produits de substitution isomères du benzène. Nitration des dibromobenzènes", in: Rec. Trav. Chim. , 1906 , 25 , pp. 191-; Full text . doi : 10.1002 / recl.19060250602
- ^ Chapter One: Studies Directed Towards a Total Synthesis of the Chartellamides. Chapter Two: Studies Directed Towards a Total Synthesis of the Chartellines . ProQuest, 2008, ISBN 0-549-77261-8 , pp. 48 ( limited preview in Google Book search).