Fluoroanilines
| Fluoroanilines | ||||||||||
| Surname | 2-fluoroaniline | 3-fluoroaniline | 4-fluoroaniline | |||||||
| other names | o -fluoroaniline | m -fluoroaniline | p -fluoroaniline | |||||||
| Structural formula |

|
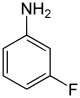
|

|
|||||||
| CAS number | 348-54-9 | 372-19-0 | 371-40-4 | |||||||
| PubChem | 9584 | 9742 | 9731 | |||||||
| Molecular formula | C 6 H 6 FN | |||||||||
| Molar mass | 111.12 g mol −1 | |||||||||
| Physical state | liquid | |||||||||
| Melting point | −35 ° C | −1 ° C | ||||||||
| boiling point | 182-183 ° C | 186 ° C | 187 ° C | |||||||
|
pK s value (of the conjugate acid BH + ) |
3.20 | 3.57 | 4.65 | |||||||
|
GHS labeling |
|
|
|
|||||||
| H and P phrases | 226-302-315-318-335 | 302-315-318-335 | 302-314 | |||||||
| no EUH phrases | see above | no EUH phrases | ||||||||
| 261-280-305 + 351 + 338 | 261-280-305 + 351 + 338 | 280-305 + 351 + 338-310 | ||||||||
In chemistry, the fluoroanilines form a group of substances that are derived from both aniline and fluorobenzene . The structure consists of a benzene ring with attached amino group (-NH 2 ) and fluorine (-F) as substituents . Their different arrangements ( ortho , meta or para ) result in three constitutional isomers with the empirical formula C 6 H 6 FN.
properties
The introduction of the fluorine atom changes the boiling point only slightly compared to aniline (184 ° C).
Individual evidence
- ↑ a b c CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b data sheet 2-fluoroaniline from Sigma-Aldrich , accessed on April 29, 2011 ( PDF ).
- ↑ a b Data sheet 3-Fluoroaniline from Sigma-Aldrich , accessed on April 29, 2011 ( PDF ).
- ↑ a b Data sheet 4-Fluoroaniline from Sigma-Aldrich , accessed on April 29, 2011 ( PDF ).
- ^ Entry on aniline in the GESTIS substance database of the IFA , accessed on December 27, 2014(JavaScript required) .


