Phenetidine
| Phenetidine | |||||||||
| Surname | 2-phenetidine | 3-phenetidine | 4-phenetidine | ||||||
| other names |
o -phenetidine, 2-ethoxyaniline, o -aminophenetol |
m -phenetidine, 3-ethoxyaniline, m -aminophenetol |
p -phenetidine, 4-ethoxyaniline, p -aminophenetol |
||||||
| Structural formula |

|
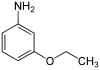
|

|
||||||
| CAS number | 94-70-2 | 621-33-0 | 156-43-4 | ||||||
| PubChem | 7203 | 12120 | 9076 | ||||||
| ECHA InfoCard | 100.002.143 | 100.009.711 | 100.005.324 | ||||||
| Molecular formula | C 8 H 11 NO | ||||||||
| Molar mass | 137.18 g mol −1 | ||||||||
| Physical state | liquid | ||||||||
| Brief description | colorless to brown liquid with an aromatic odor |
yellowish near odorless liquid |
colorless to yellowish liquid with a characteristic odor |
||||||
| Melting point | −4 ° C | - | 3 ° C | ||||||
| boiling point | 232-234 ° C | 248 ° C | 254 ° C | ||||||
|
pK s value (of the conjugate acid BH + ) |
4.43 | 4.17 | 5.20 | ||||||
| solubility | 7 g l −1 (20 ° C) | poorly soluble | 20 g l −1 (20 ° C) | ||||||
|
GHS labeling |
|
|
|
||||||
| H and P phrases | 301-311-331-373 | 301 + 311 + 331-373 | 341-302 + 312-331-317-319-412 | ||||||
| no EUH phrases | no EUH phrases | no EUH phrases | |||||||
|
280-302 + 352 304 + 340-309 + 310 |
280-261-301 + 310-311 | 280-260-301 + 310-314-501 | |||||||
The phenetidines (also ethoxyanilines or aminophenetols ) form a group of substances that are derived from both phenetol (ethoxybenzene) and aniline . The structure consists of a benzene ring with attached ethoxy (–OCH 2 CH 3 ) and amino groups (–NH 2 ) as substituents . Their different arrangement results in three constitutional isomers with the empirical formula C 8 H 11 NO. Primarily they can be viewed as ethoxy substituted anilines.
Just as the anis idines are derived from the root anis ol , the naming of the ethoxy derivatives as phenet idines proceeding from phenet ol in an analogous manner .
properties
The phenetidines have (4.603) only slightly different pK opposite the aniline s values.
Extraction and presentation
2- and 4-phenetidine are produced from phenetol by nitration (to 2- or 4-nitrophenetol ) and subsequent hydrogenation .
Individual evidence
- ↑ a b c d Entry on 2-phenetidine in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c d Entry on 3-phenetidine in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c d Entry on 4-phenetidine in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Entry on Phenetidine. In: Römpp Online . Georg Thieme Verlag, accessed on December 17, 2014.


