Chlorobenzotrichloride
| Chlorobenzotrichloride | ||||||||
| Surname | 2-chlorobenzotrichloride | 3-chlorobenzotrichloride | 4-chlorobenzotrichloride | |||||
| other names |
o -chlorobenzotrichloride α, α, α, 2-tetrachlorotoluene, 2-chloro-1- (trichloromethyl) benzene |
m -chlorobenzotrichloride α, α, α, 3-tetrachlorotoluene, 3-chloro-1- (trichloromethyl) benzene |
p -chlorobenzotrichloride α, α, α, 4-tetrachlorotoluene 1-chloro-4- (trichloromethyl) benzene |
|||||
| Structural formula |

|

|
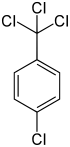
|
|||||
| CAS number | 2136-89-2 | 2136-81-4 | 5216-25-1 | |||||
| PubChem | 16494 | 75052 | 21277 | |||||
| ECHA InfoCard | 100.016.707 | 100.016.706 | 100.023.645 | |||||
| Molecular formula | C 7 H 4 Cl 4 | |||||||
| Molar mass | 229.92 g mol −1 | |||||||
| Physical state | firmly | ??? | liquid | |||||
| Brief description | colorless, flammable solid with a pungent odor |
??? | colorless liquid with a characteristic odor |
|||||
| Melting point | 29−31 ° C | ??? | 5.8 ° C | |||||
| boiling point | 260-264 ° C | 245 ° C | ||||||
| density | 1.508 g cm −3 (25 ° C) | 1.49 g cm −3 (25 ° C) | ||||||
| solubility | Decomposes in water, soluble in ether, acetone |
??? | Decomposes in water, soluble in alcohol, ether, acetone |
|||||
| Flash point | 98 ° C | 113 ° C | ||||||
| Ignition temperature | 505 ° C | |||||||
|
GHS labeling |
|
|
|
|||||
| H and P phrases | 302-315-319-335-351-413 | see above | 302-312-315-335-350-361f-372 | |||||
| see above | see above | no EUH phrases | ||||||
| ? | see above | 201-261-280-308 + 313 | ||||||
The chlorobenzotrichlorides form a group of substances that can be understood as halogenated benzotrichlorides . The different arrangement of the substituents results in three constitutional isomers with the empirical formula C 7 H 4 Cl 4 .
presentation
4-chlorobenzotrichloride is formed from a mixture of 4-chlorobenzotrifluoride and aluminum trichloride (anhydrous) with subsequent addition of silicon tetrachloride ; the reaction mixture is distilled in vacuo.
use
Chlorobenzotrichlorides are used as intermediates in the manufacture of herbicides, dyes, disinfectants and pharmaceuticals.
Individual evidence
- ↑ a b c d e f g Entry on 2-chlorobenzotrichloride in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b c d e f g h Entry on 4-chlorobenzotrichloride in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ Data sheet 4-Chlorobenzotrichloride from Sigma-Aldrich , accessed on April 27, 2011 ( PDF ).
- ↑ Process for the preparation of nuclear halides of benzotrichlorides from the corresponding benzotrifluorides .
- ↑ Toxicological assessment of p-chlorobenzotrichloride (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ↑ Toxicological assessment of o-chlorobenzotrichloride (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.

