Bischler-Möhlau indole synthesis
The Bischler Möhlau indole synthesis is a reaction from the field of organic chemistry and named after the chemists August Bischler and Richard Möhlau . It describes the representation of indole and its derivatives .
Overview
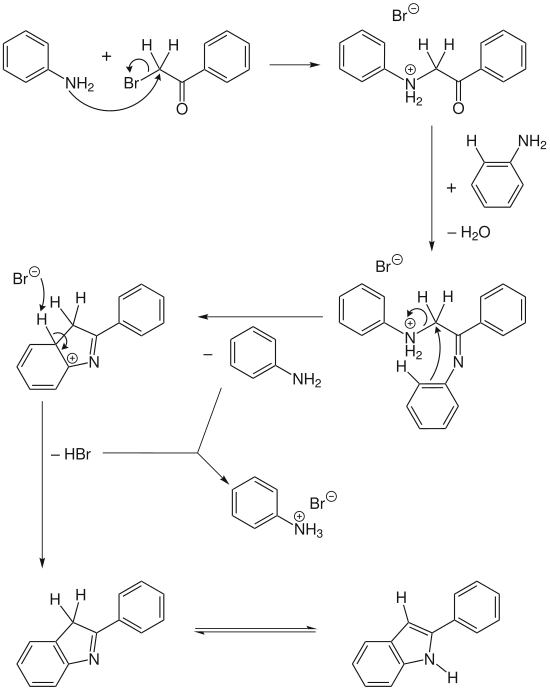
ω-Bromoacetophenone can be converted to 2-arylindole with aniline in excess . This releases water and aniline hydrobromide.
Despite the history of this classic reaction, it receives relatively little attention compared to other indole syntheses. The reason may lie in the relatively harsh reaction conditions in contrast to other reactions. Much milder methods have been developed later, such as the use of lithium bromide as a catalyst or the use of microwaves .
Reaction mechanism
Although simple starting materials are used, the reaction mechanism is relatively complex.
In two steps, a charged aniline is formed as an intermediate product from two molecules of aniline and one molecule of ω-bromoacetophenone with elimination of water. This can cyclize electrophilically and form an intermediate. The desired indole is created through aromatization and subsequent tautomerism . One split off molecule each of aniline and hydrogen bromide form the aniline hydrobromide, a salt from the class of hydrobromides .
Other indole syntheses
Individual evidence
- ↑ Aug. Bischler: On the Origin of Some Substituted Indoles. In: Reports of the German Chemical Society. 25, No. 2, 1892, pp. 2860-2879, doi : 10.1002 / cber.189202502123 .
- ↑ Aug. Bischler, P. Fireman: On the knowledge of some α-β-diphenylindoles. In: Reports of the German Chemical Society. 26, No. 2, 1893, pp. 1336-1349, doi : 10.1002 / cber.18930260232 .
- ↑ Richard Möhlau: About the action of primary aromatic amine bases on acetophenone bromide. In: Reports of the German Chemical Society. 14, No. 1, 1881, pp. 171-175, doi : 10.1002 / cber.18810140146 .
- ↑ Richard Möhlau: About Diphenyldiisoindol. In: Reports of the German Chemical Society. 15, No. 2, 1882, pp. 2480-2490, doi : 10.1002 / cber.188201502204 .
- ^ Emil Fischer , Theodor Schmitt: Ueber Pr-2-Phenylindol. In: Reports of the German Chemical Society. 21, No. 1, 1888, pp. 1071-1077, doi : 10.1002 / cber.188802101200
- ↑ Karin Pchalek, Ashley W. Jones, Monique MT Wekking, David StC. Black: Synthesis of activated 3-substituted indoles: an optimized one-pot procedure. In: Tetrahedron. 61, No. 1, 2005, pp. 77-82, doi : 10.1016 / j.tet.2004.10.060 .
- ↑ Vellaisamy Sridharan, Subbu Perumal, Carmen Avendaño, J. Carlos Menéndez: Microwave-Assisted, Solvent-Free Synthesis Bishler indoles. In: Synlett. No. 1, 2006, pp. 0091-0095, doi : 10.1055 / s-2005-922760 .