Davidson oxazole cyclization
The Davidson oxazole cyclization or Davidson oxazole synthesis is a name reaction in organic chemistry that is named after David Davidson. The reaction describes the synthesis of a substituted oxazole from an O- acylacyloin. When making the discovery, Davidson took up the research of Francis Japp from 1883 and published detailed studies in 1937.
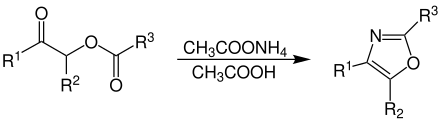
Overview reaction
O -Acylacyloin reacts with the use of ammonium acetate and acetic acid to form substituted oxazole:
Reaction mechanism
The reaction mechanism is described in the literature:
The acyloin derivative 1 reacts with ammonium acetate, which is converted into acetic acid, to form the ionic intermediate 2 . The isomeric intermediate 3 is then formed by reaction with acetic acid . Electron and atom rearrangement creates a ring closure - intermediate stage 4 is created . The protonated oxazole intermediate 5 is obtained by splitting off water twice . Under the influence of a base, deprotonation takes place and the oxazole 6 is formed .
application
In a Davidson-oxazole cyclization, bromoacetophenone reacts with sodium formate to form 2-phenyloxazole:
Individual evidence
- ↑ D. Davidson, M. Weiss and M. Jelling: THE ACTION OF AMMONIA ON BENZIL In: J. Org. Chem. 02 (4), 1937, pp. 319-327, doi: 10.1021 / jo01227a004 .
- ↑ D. Davidson, M. Weiss and M. Jelling: THE ACTION OF AMMONIA ON BENZOIN In: J. Org. Chem. 02 (4), 1937, pp. 328-334, doi: 10.1021 / jo01227a005 .
- ^ Z. Wang: Comprehensive organic name reactions and reagents Volume 1 . John Wiley, Hoboken (NJ) 2009, ISBN 978-0-470-28662-3 , pp. 855-857 .
- ↑ Scott E. Whitney, Michael Winters and Bruce Rickborn: Benzyne-oxazole cycloadducts: isolation and retro-Diels-Alder reactions In: J. Org. Chem. 55 (3), 1990, pp. 929-935, doi: 10.1021 / jo00290a025 .