Radziszewski synthesis
The Radziszewski synthesis is a name reaction of organic chemistry and named after the Polish chemist Bronisław Leonard Radziszewski (1838–1914). It describes the preparation of imidazole and its derivatives . For this purpose, a 1,2- diketone is reacted with ammonia and an aldehyde .
Reaction mechanism
The corresponding imidazole is prepared from 1,2-di ketones , ammonia and an aldehyde . This mechanism takes place in two main reaction steps, which are described below.
First step
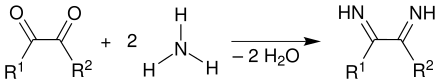
Ammonia reacts with the carbonyl groups of the diketone to form a diimine , i.e. a Schiff base, with elimination of water .
Second step
The imine then attacks the carbonyl group of the aldehyde. The desired imidazole is formed with elimination of water.
Individual evidence
- ↑ B. Radziszewski, Reports of the German Chemical Society , 1882 , 15, 1493.
- ↑ B. Radziszewski, Reports of the German Chemical Society , 1882 , 15, 2706.
- ^ Z. Wang: Comprehensive Organic Name Reactions and Reagents Volume 3, Wiley Verlag, 2009, p. 2293, ISBN 978-0-471-70450-8 , (3-volume set).