Graebe-Ullmann synthesis
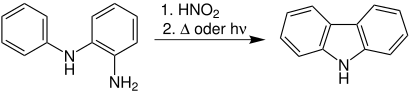
The Graebe-Ullmann synthesis , often referred to as Grabe-Ullmann thermolysis or Grabe-Ullmann carbazole synthesis , is a name reaction in organic chemistry . It was published for the first time in 1896 by the German chemists Carl James Peter Graebe (1841-1927) and Fritz Ullmann (1875-1939). With their help you can make carbazoles .
The reaction can take place thermally, photochemically or under the influence of microwave radiation.
mechanism
The illustration shows the mechanism postulated by Zerong Wang using the example of the reaction of ortho- aminodiphenylamine to carbazole:
First , the primary amino group in the diphenylamine derivative 1 is diazotized with nitrous acid . This gives the diazonium salt 2 , which cyclizes to the cation 3 through an intramolecular attack . The triazole 4 is obtained by splitting off a proton . From this nitrogen is now split off and radical 5 is formed . A radical rearrangement turns 5 into the carbene intermediate 6 . A cyclization then takes place, in which the carbazole derivative 7 is obtained, which aromatizes to carbazole ( 8 ) via a [1,3] proton transfer .
Analyzes have shown that this reaction involves the cyclization of a carbene to a carbazole derivative. It is also known that the carbazole derivative reacts to carbazole via a [1,3] proton transfer. On the basis of these known facts, the presented mechanism could be deduced. With the help of this reaction it is also possible to use more complex heterocyclic compounds - z. B. Pyrrole derivatives - to synthesize.
example
The following example is intended to show the practical applicability of the Graebe-Ullmann synthesis:
The reaction shows the Graebe-Ullmann synthesis of a quinoline derivative. Instead of the phenyl ring shown here, there can also be other radicals on the triazole ring.
Individual evidence
- ↑ a b c after: Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 1 Volume Set . John Wiley & Sons, Hoboken, New Jersey, 2009, ISBN 978-0-471-70450-8 , pp. 1256-1259.
- ↑ C. Graebe, F. Ullmann: About a new carbazole synthesis. In: Justus Liebig's Annals of Chemistry. 291, 1896, pp. 16-17, doi: 10.1002 / jlac.18962910104 .
- ↑ Andrés Molina et al: Synthesis and DNA Binding Properties of γ-Carbolinium Derivatives and Benzologues . In: The Journal of Organic Chemistry . tape 61 , no. 16 , 1996, pp. 5587-5599 , doi : 10.1021 / jo960266h .
- ^ Alan R. Katritzky, Xiangfu Lan, Jason Z. Yang, Olga V. Denisko: Properties and Synthetic Utility of N-Substituted Benzotriazoles . In: Chem. Rev. Band 98 , no. 2 , 1998, p. 409-548 , doi : 10.1021 / cr941170v .
- ↑ a b Johnson Stanslas et al .: antitumor Polycyclic Acridines. 7.1 Synthesis and Biological Properties of DNA Affinic Tetra- and Pentacyclic Acridines . In: J. Med. Chem. No. 43 , 2000, pp. 1563-1572 , doi : 10.1021 / jm9909490 .