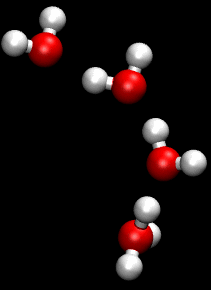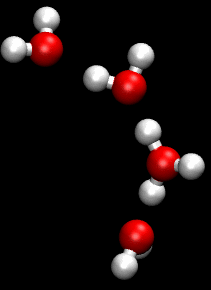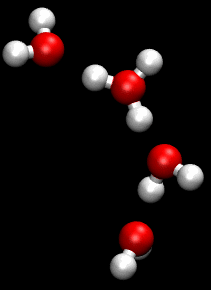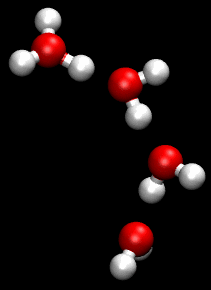
Grotthuss mechanism
The Grotthuss mechanism , described by Theodor Grotthuss , has the consequence that protons and hydroxide ions in aqueous solution supposedly “migrate” faster than other ions in an electric field. They therefore have a greater conductivity than other ions.
This mechanism is a chain mechanism: instead of transporting protons through the solution, bonds and hydrogen bonds are broken and re-established. This enables the bindings to be “folded down” and the load to be passed on very quickly.
Example table for the conductivity of selected cations
| cation | Conductivity / cm 2 V −1 s −1 |
|---|---|
| NH 4 + | 0.763 × 10 −3 |
| Na + | 0.519 × 10 −3 |
| K + | 0.762 × 10 −3 |
| H + | 3.62 × 10 −3 |
See also
literature
- Peter W. Atkins: Short textbook physical chemistry . Wiley-VCH, Weinheim 2001, ISBN 3-527-30433-9 ( limited preview in the Google book search), p. 382.