Retarder (polymerization)
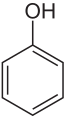
Retarders , also called inhibitors or stabilizers, are substances that in the plastics industry delay or even prevent the polymerization ( radical chain polymerization ) of monomers. This is achieved in particular by substances that easily form radicals through a transfer reaction . The resulting radicals are inert and do not react to any significant extent with the monomers. Aromatic compounds such as phenol, hydroquinone or 4-nitrophenol are usually used as retarders :
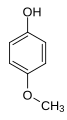
4-methoxyphenol / hydroquinone monomethyl ether / HQME
Mode of action
The effect of the retarder is based on the formation of low-energy, mesomeric-stabilized radicals. The unpaired electron of the radical is stabilized by resonance with the π electrons of the aromatic.
application
In the case of monomers, retarders should increase the storage stability and prevent undesired premature polymerization during processing. Before the desired polymerization process, the retarder must be separated from the monomer. This is done either by distillation or by adsorption . Sometimes the effect of the retarder is simply compensated (“overrun”) by an excess of catalyst. The radical chain polymerization only starts when the retarder is “used up”, i.e. after a time delay ( induction period ).
Individual evidence
- ↑ Karlheinz Biederbick: Kunststoffe , 4th edition, Vogel-Verlag, Würzburg, 1977, pp. 45–46, ISBN 3-8023-0010-6 .