Walden reversal
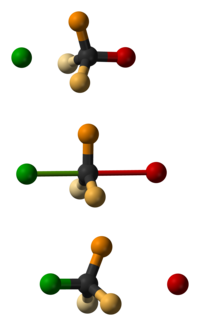
As a Walden reversal (or Walden reversal , sometimes also umbrella mechanism ) one describes the inversion (change) of the configuration at a stereochemical center, usually a carbon atom , within a chemical reaction . The mechanism was named after the Latvian-German chemist Paul Walden , who published his discovery in 1896.
Reaction course
During an S N 2 reaction, the following steps occur in concert: the entry group approaches the central atom, the leaving group moves away from the central atom and the three substituents of the central atom not directly involved in the reaction pass through a planar transition state . If one considers the entry and leaving group, a trigonal-bipyramidal transition state is present, in which the nucleophile and leaving group are in the axial positions. Similar to folding an umbrella, the resulting stereochemical arrangement of the substituents on the reaction product is different from that of the starting material.
Whether the change in the arrangement of the substituents is also accompanied by a change in the absolute stereochemical configuration depends on the prioritization of the four residues according to the Cahn-Ingold-Prelog system . If the entering and leaving group have the same priority in the order of precedence, the stereochemical descriptor changes, for example from ( R ) - to ( S ) -.
literature
- Peter Sykes: Reaction Mechanisms in Organic Chemistry. 9th edition, Verlag Chemie 1979, ISBN 3-527-26872-3 .
Individual evidence
- ↑ P. Walden: About the mutual transformation of optical antipodes. Reports of the German Chemical Society 1896, 29, 133-138. doi : 10.1002 / cber.18960290127