Ivermectin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Ivermectin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
white to yellowish, crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
155 ° C (mixture) |
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ivermectin is an effective substance against ectoparasites (lice, mites, ticks) and roundworms ( nematodes ).
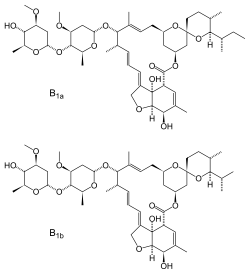
Chemically, Ivermectin is one of the macrocyclic lactones ( macrolides ) from the group of avermectins , which are metabolic products of Streptomyces avermitilis . Ivermectin is a mixture of two different semi-synthetic avermectins, which have the spectrum of activity of avermectin B 1 and B 2 and are referred to as H 2 B 1a (90% share) and H 2 B 1b (about 10% share).
Ivermectin is mainly used in veterinary medicine as a medicinal substance for the treatment and prevention of infectious diseases ( parasitosis , helminthiasis ) caused by ectoparasites or roundworms .
In 2015 the Nobel Prize in Physiology or Medicine was awarded to the American William C. Campbell and the Japanese Satoshi Ōmura for the development of ivermectin.
discovery
The discovery of avermectins goes back to a soil sample taken in Japan in 1974, which contained the microorganism Streptomyces avermitilis . Ōmura sent several thousand fermentation broths to Merck Research Laboratories , where Campbell found the strong anthelmintic activity of avermectin B 1 and its derivative ivermectin.
In 1981, clinical trials began. In 1987, Ivermectin was approved under the trade name Mectizan . The then Chairman of the Board of Management of MSD , P. Roy Vagelos , decided to provide Mectizan free of charge to the developing world because of its effectiveness against river blindness .
Mechanism of action
Ivermectin is liposoluble and is quickly absorbed and distributed in the body when administered orally, parenterally and through the skin. It accumulates in the liver and fatty tissue and is slowly released from there. It is excreted in the bile and then in the feces. Small amounts are also excreted in the urine and milk .
Ivermectin binds to the glutamate- activated chloride channels, which only occur in invertebrates, and to γ-aminobutyric acid -activated chloride channels. The resulting induced influx of chloride - ions in the cell leads to hyperpolarization of the cell membrane, the excitation transition blocked. This leads to paralysis and ultimately death of the parasites. In addition, the worms' egg formation and larval development are disrupted.
In ticks , egg production and molting are inhibited and the reproductive cycle is disrupted, but the tick itself does not fall off the host.
In vertebrates Ivermectin acts generally less toxic, as the main active target - glutamate-activated chloride channels - among them does not exist. However, ivermectin at higher concentrations activates u. a. also inhibitory GABA A and glycine receptors , such as those found in the central nervous system of vertebrates (including humans). This can lead to severe CNS depression and, in extreme cases, death. Toxicity also depends on how well ivermectin can cross the blood-brain barrier of the species. In mammals , the permeability is usually low.
Spectrum of activity and application
Ivermectin is effective against all clinically important roundworms (nematodes). In addition, the agent is effective against lice , mites ( Psoroptes spp., Sarcoptes ssp., Otodectes cynotis , Demodex ssp., Knemidocoptes ssp., Red bird mite , Nordic bird mite , air sac mites , etc.), rabbit fleas , botflies ( Dermatobia , Hypoderma ), sheep louse flies ( Melophagus ) and microfilariae .
Tapeworms (Trematoda and Cestoda) are immune to ivermectin, but sensitive to the active ingredient praziquantel , which is contained in some veterinary combination preparations (deworming pastes and tablets). Initial resistance is also suspected in some roundworms .
In humans, ivermectin is used as a single oral dose and as a topical treatment for rosacea and scabies. In Germany, approval for human diagnoses only exists for the treatment of rosacea and scabies, so other treatments should only be carried out according to the strictest indication by a doctor. It should be taken on an empty stomach with water with an interval of two hours before the first meal. To avoid recurrence , all contact persons must be treated at the same time. The side effects known from the therapy of onchocerciasis (river blindness) are based on the massive death of the microfilariae and are not to be expected in the case of mite infestation. Oral therapy is particularly recommended for eyelashes (e.g. pubic lice), as the Z. topically applied agents can damage the cornea . The agent is contraindicated during pregnancy and breastfeeding, as well as in children under 5 years of age .
In 2012, a 0.5% ivermectin lotion was approved in the USA for single use in the scalp hair, which is an effective therapy for head lice . The application is low-risk, tested from the age of six months and is also suitable for lice with resistance to other agents.
In animals (pets, birds, reptiles) the agent is administered orally , subcutaneously , intramuscularly or through the skin. Repeat after a week is recommended for many parasites.
Contraindications and side effects
In collies , collie mongrels, Shelties , Bobtails and related breeds Ivermectin should not be used orally, since with them due to frequent genetic defect ( MDR1 defect deaths may occur). Young rats, hedgehogs, turtles , chameleons and small lizards are also very sensitive. In crocodiles , the application is contraindicated. There were more deaths in finches . Ivermectin should also not be used in very young animals.
Local reactions may occur during administration (swelling of the injection site or skin irritation in the case of local administration).
To date, hypersensitivity reactions ( anaphylaxis ) have only been observed in horses and dogs . Edema can occur in horses . In dogs, the massive death of microfilariae can cause shock six hours after treatment .
Environmental damage
Since the use of ivermectin damages the coprophage fauna of treated livestock, some animal species are deprived of their food sources. These include, for example, the Chough ( Pyrrhocorax pyrrhocorax ), which in many places mainly feeds on larvae from sheep droppings. The diet of these birds are numerous types of beetles and flies, which specialize in breaking down animal excrement, often only from certain species. This directly threatens their existence. Furthermore, the manure is no longer broken down as quickly, which leads to production losses, especially in horse and cattle pastures, as the grass cannot grow back under the long-lying manure.
Chemical information
The white to whitish-yellow, crystalline substance is stable in the crystalline state, but is sensitive to light in polar solvents. Ivermectin is practically insoluble in water, but dissolves well in methanol , ethanol , chloroform , acetone, and tetrahydrofuran .
The two components of ivermectin H 2 B 1a and H 2 B 1b differ structurally only by one methyl group . They arise through selective hydrogenation of the cis -configured 22,23 bond from avermectin B 1a and avermectin B 1b .
The letters and numbers in the names of the components characterize certain avermectin structures such as double or single bonds between C -22 and C-23 with / without substituents on C-23 (1 or 2), as well as substituents on C-5 (A or B) and on the C-25 (a or b). The molecule has 19 chiral centers.
Trade names
- Human medicine: Scabioral and Driponin (D), Scaboral (NL), Soolantra, Stromectol
- Veterinary medicine: Agrimec, Animec, Bimectin, Closamectin, Ecomectin, Eraquell, Equimax, Eqvalan, Furexel, Ivomec, Noromectin, Otimectin, Paramectin, Qualimec, Vectin, Virbamec
SARS-CoV-2 research
Ivermectin has been given in some countries to inhibit the replication of SARS-CoV-2. In doing so, they relied on publications withdrawn in June 2020, which were based on a large patient database of invented data.
Web links
- Entry on Ivermectin at Vetpharm, accessed August 11, 2012.
Individual evidence
- ↑ a b c d e Data sheet for the drug Stromectol / Ivermectin from Merck (USA), (PDF; 66 kB), accessed on February 17, 2010.
- ↑ a b Data sheet Ivermectin from Sigma-Aldrich , accessed on April 6, 2011 ( PDF ).
- ^ Jan Osterkamp: A Nobel Prize for Medicine. Spektrum.de, October 5, 2015.
- ↑ David Molyneux, Hugh R. Taylor: The discovery of ivermectin . In: Trends in Parasitology . tape 31 , no. 1 , 2015, p. 1 , doi : 10.1016 / j.pt.2014.10.003 .
- ^ Ivermectin History
- ↑ Mectizan Donation Program
- ↑ A. Estrada-Mondragon, JW Lynch: Functional characterization of ivermectin binding sites in α1β2γ2L GABA (A) receptors . In: Frontiers in molecular neuroscience , 2015, 8, p. 55. doi: 10.3389 / fnmol.2015.00055 , PMC 4585179 (free full text)
- ↑ Talcott, Patricia A. Small animal toxicology . 2nd ed. Saunders / Elsevier, St. Louis, Mon. 2006, ISBN 978-0-7216-0639-2 ( limited preview in Google book search).
- ↑ Ivermectin: An Insecticide Against Rosacea. In: Pharmaceutical newspaper online. May 12, 2015, accessed on September 17, 2017 (approval for Soolantra in rosacea from May 1, 2015).
- ↑ Oral ivermectin available in Germany. In: Pharmaceutical newspaper. 2016, accessed September 17, 2017 .
- ↑ David M. Pariser, Terri Lynn Meinking, Margie Bell, William G. Ryan: Topical 0.5% Ivermectin Lotion for Treatment of Head Lice . In: New England Journal of Medicine . tape 367 , no. 18 , November 1, 2012, p. 1687-1693 , doi : 10.1056 / NEJMoa1200107 .
- ↑ Urs N. Glutz von Blotzheim, KM Bauer: Handbuch der Vögel Mitteleuropas. Volume 13 / III: Passeriformes (4th part). AULA-Verlag, Wiesbaden 1993, ISBN 3-89104-460-7 , p. 1632.
- ↑ K. Hardtke et al. (Ed.): Commentary on the European Pharmacopoeia Ph. Eur. 5.0, Ivermectin. Loose-leaf collection, 19th delivery 2005, Wissenschaftliche Verlagsgesellschaft Stuttgart.
- ↑ Infectopharm: Instructions for use Scabioral. (PDF) (No longer available online.) In: Instructions for use. Infectopharm, May 2016, archived from the original on February 15, 2017 ; accessed in May 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Julia Köppe: Alleged Corona database: Fatal trust. In: Spiegel online. Retrieved June 14, 2020 .